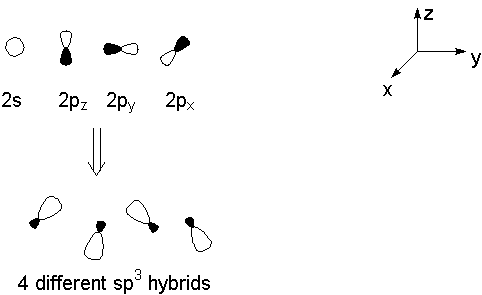
Two major approaches exist for describing bonding in organic chemistry. Both begin with the orbital theory of atomic electronic structure: that in isolated atoms, electrons behave according to mathematical descriptions called "orbitals." The wavefunctions for these orbitals may be permuted by addition or subtraction to favor placing the electrons in specific regions of space. If we do this for a single atom, the result is the familiar "hybrid orbitals:" for carbon, these may be sp, sp2, or sp3.

The valence bond approach tells us to assess the geometry of the molecule, and locate where the electrons need to be in space to decide how to hybridize each atom. Bonds will form by sharing electrons from atom whose hybrid orbitals occupy the same region of space. For example, ethylene is a planar molecule, with bond angles of approximately 120°. The best hybridization for this geometry is to form sp2 hybrid orbitals to use for making C-C and C-H bonds:
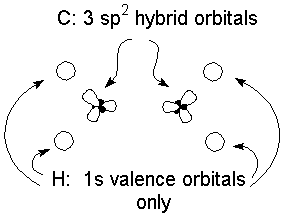
An important result of this analysis is that by using the 3 sp2 hybrids, each carbon has a remaining unused 2p orbital (perpendicular to the plane of the molecule) and an unused valence electron. Sharing these electrons results in the formation of a second component of the double bond:
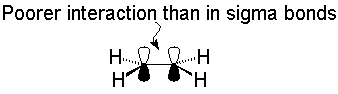
Most organic molecules have geometries that closely match the bond angles predicted by sp, sp2 or sp3 hybridization, and the bond-line notation presumes that a single bond is formed from overlap of hybrid orbitals to form a sigma bond, while the additional bonding components of multiple bonds are formed from pi-type overlap of p orbitals.

Forward to MO theory
Forward to resonance and delocalized bonding
Back to CH 630 Home page
Last updated: 09/21/2000