Vacuum distillation
(ref: Mohrig, Sec. 12.7, pp. 197-202)
Two slightly different setups, and two different vacuum sources, will be used for this
part of the experiment. The relationship between applied pressure and boiling point (taken
from student data) is available here.
The combinations:
(Rotary evaporators will be used to remove ether; they
also use the principle of vacuum distillation but are slightly different in their design.)
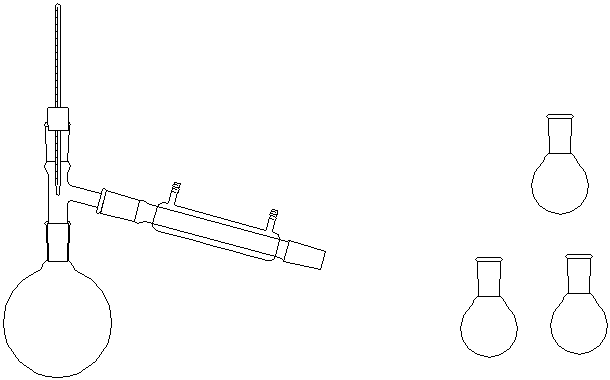
| A view of the apparatus used for an aspirator distillation: (The receiving flasks
on the cow have been omitted for clarity, as has the mercury manometer.) |
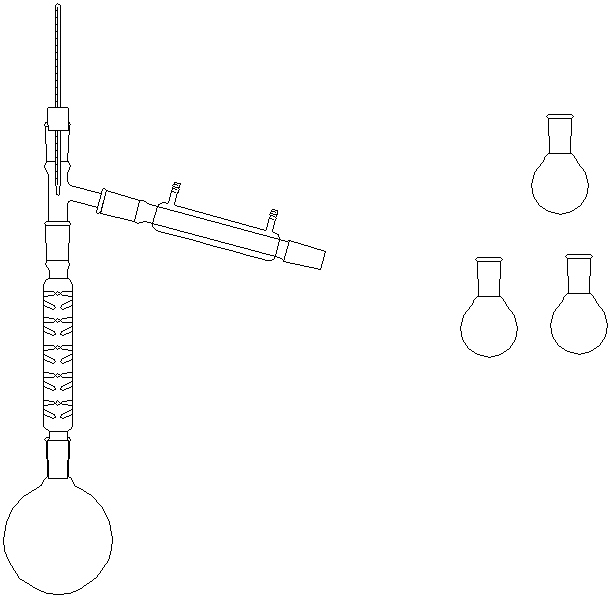
A view of the apparatus used for a vacuum pump distillation: (Note that in
distillation of the P-ester, a distilling column is placed between the Claisen adapter and
the still head.) |
 |
 |
Both of these need to be wrapped with glass wool and aluminum foil for insulation.
When assembling them, build from the bottom up!
Note: Use of the Claisen adapter is
recommended when additional volume is needed for solutions that foam or "bump."
However, its use requires more time, as you must warm up all the glass in the
adapter. It is necessary for
distillation of the H-ester; also, make sure you use a 100-ml flask as the still pot.
It is not necessary for distillation of the P-ester,
as you will use a packed column on top of the still pot.
Click here
to see diagrams of the individual pieces.
Glassware for vacuum distillation of your
compounds:
You will need:
- A 100-ml, 1-neck round bottom flask
- A distilling head
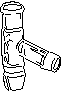
- Thermometer adapter (and thermometer) Note: make sure the rubber seal is not
cracked or dried out. If it is, exchange it for a good one at the issue room.



- Collection flasks, 25-50 mL (Make sure these have glass or wire hooks. Tare them and
record their masses before assembling!)
- A stir bar or boiling chips
For the distillation of the ester, you will need the same
equipment; those distilling the butyl cyclopropanecarboxylate will also need a packed
distilling column (check out from the issue room) 
Vacuum sources:
Water aspirator
The principle of operation is the Venturi principle: motion of water past a
narrow opening creates low vacuum.
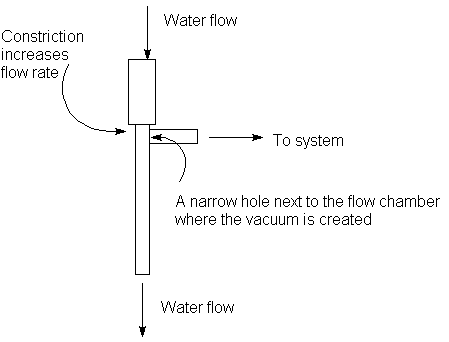
There are several aspects to its operation to remember:
- Have the maximum possible water flow (for the optimum vacuum).
- Changes in water flow will cause a change in the ultimate vacuum generated.
- If you turn off the water before venting, the water will back up into the sysytem.
Mechanical vacuum pump
The principle of operation is to have an enclosed cylindrical chamber in which an
offset flywheel with two spring-loaded vanes rotates. The vacuum attainable is largely a
function of the vapor pressure of the pump oil.
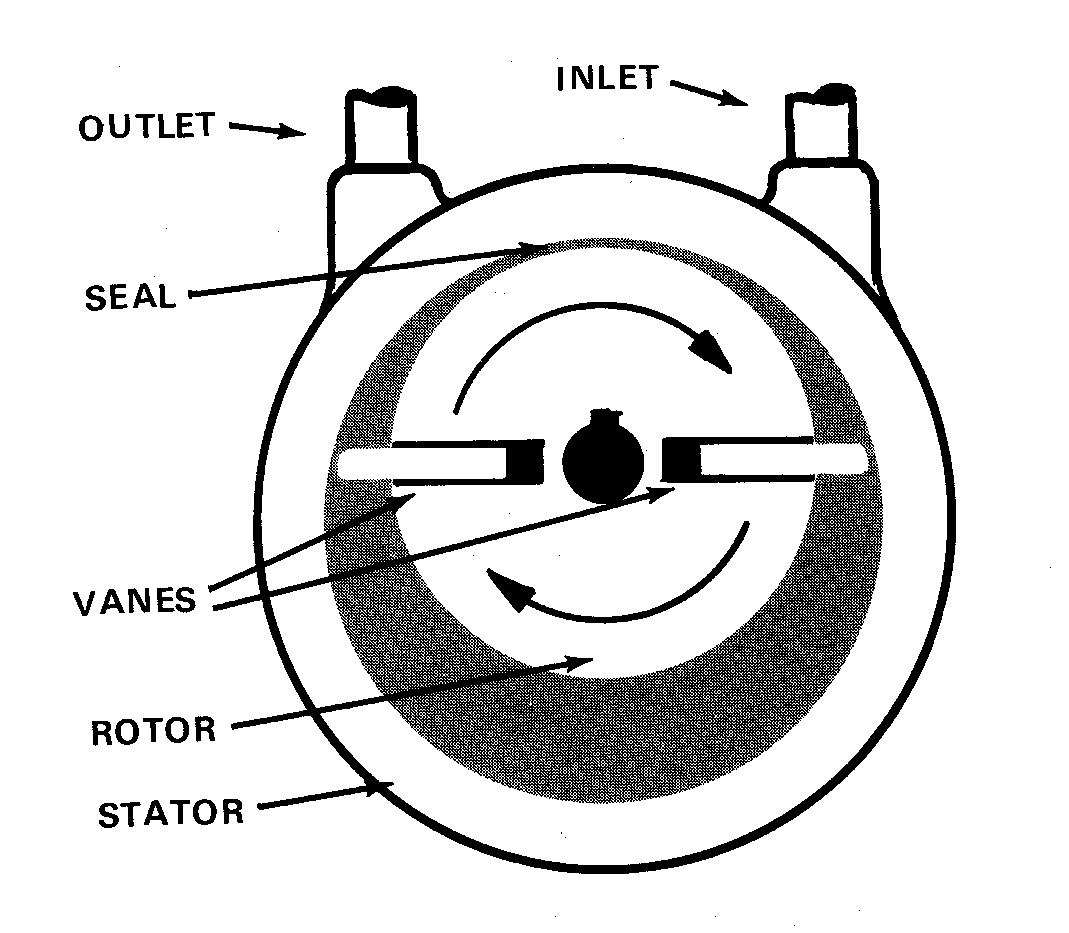
In CH 362, each two distilling stations are connected to a single mechanical pump via a
glass manifold. Each station may be isolated by closing the appropriate stopcock.
Several problems may occur.
If you don't achieve the anticipated ultimate vacuum (<10 mm Hg for a mechanical
pump, <40 mm Hg for a water aspirator), do the following:
- Isolate your apparatus from the vacuum source. If you get good vacuum with nothing
attached, then there is a leak. Carefully turn each ground glass joint to make sure a good
grease seal has been achieved.
- If there a poor vacuum with no system attached, the vacuum source has a problem. Switch
stations or ask the TA for help.
- Foaming may occur. This is normal. If you have a large enough pot flask, so that the
bubbles break before entering the distilling head, you need do nothing.
- If the foam seems to be entering the still head, close off or vent the vacuum source
until the foam subsides. Note: for distillation of the H-ester, you will need to
heat the pot more (5-10 V on the Variac) to reduce surface tension. Slowly reestablish the
vacuum.
Back to H-ester page
Back to P-ester page
Back to CH362 Home Page
Comments to K. Gable










