Pressure vs. Temperature
for Vacuum Distillation
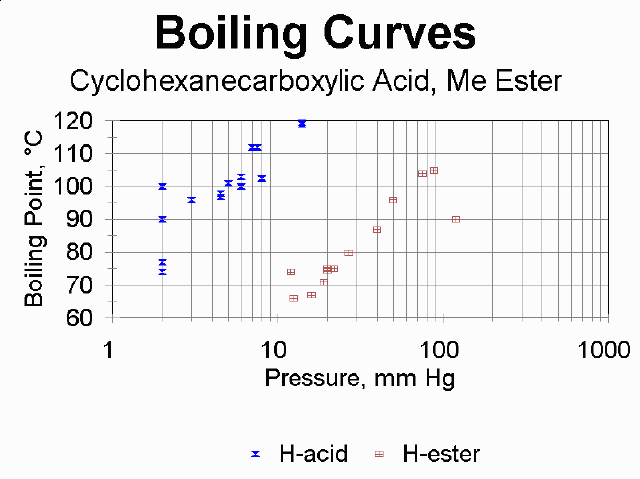
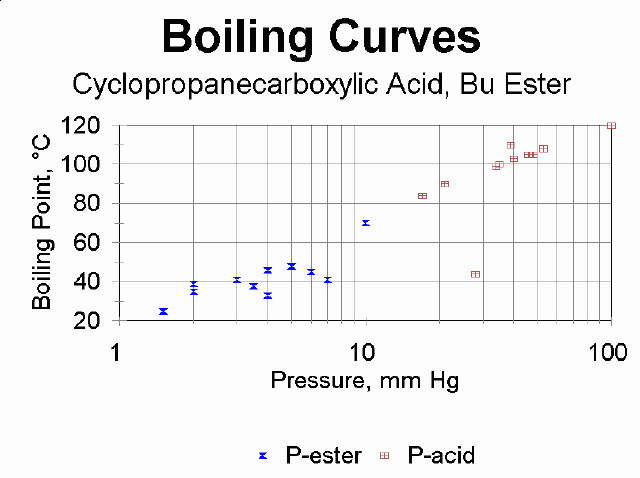
An interactive nomograph for predicting BP at a particular pressure is provided by Sigma-Aldrich. This gives you an approximate idea of where to expect your compound to distill, but this will be affected by the vapor pressure of impurities as well as the quality of how well all your joints and hose connection are sealed. See below for some actual student data from this experiment; you may wish to look up the atmospheric boiling points for the four compounds and compare predictions to observations.
The nomograph is based on ideal behavior according to the Clausius-Clapeyron equation. It is known that carboxylic acids deviate from this substantially due to the H-bonding dimerization they exhibit.
Shown are plots for data that students reported in 1998.
Back to Vacuum Distillation Page
Back to CH 362 Home Page
09/09/2002