In aromatic amines, the conjugation with the benzene ring makes the amine a worse proton acceptor, but this phenomenon is strongly affected by any substituents on the aromatic ring.
Name, pKa of Ammonium Ion, Structure |
Electrostatic PotentialLoad ESP mapsBackground: Black White |
pKa of Ammonium Ion |
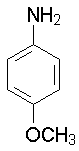
p-Methoxyaniline
5.34
|
||
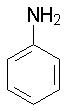
Aniline
4.63
|
||
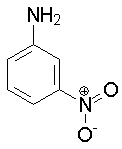
m-Nitroaniline
2.47
|
||
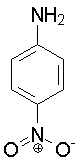
p-Nitroaniline
1.00
|
||
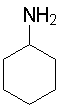
Cyclohexylamine
10.66
|
||
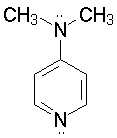
N, N-Dimethyl- |
You should be able to make several comparisons. First, what effect does the functional group (methoxy, nitro) have on the basicity of the anilines? Why is cyclohexylamine a better base than the anilines?
Finally, where do you think dimethylaminopyridine will be protonated? Why?
Back to Page 1.