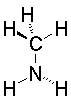
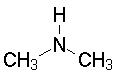
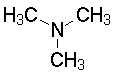
The lone pair on nitrogen is an excellent proton acceptor. Compare the structures and pKas of several simple amines (We use the pKa of the conjugate acid ammonium salt to measure basicity; can you explain why?).
Look at how localized the electron density is on nitrogen. Also look at how pyramidal the nitrogen is.
Name, pKa of Ammonium Ion |
Electrostatic PotentialLoad ESP mapsBackground: Black White |
Structure |
Methylamine10.66
|
Show angle measurements All of these are near the ideal 109.5° needed for an sp3 nitrogen. |
|
Dimethylamine10.73
|
Show angle measurements Note that the C-N-C angle opens up a bit, due to steric compression. (Look at a spacefilling model.) |
|
Trimethylamine9.81
|
Show angle measurements Now the sum of the angles has increased to 134°; this molecule is more planar. Nitrogen has shifted toward sp2 hybridization; it is less basic. A planar nitrogen (if we could make it) would be even less basic. |
Trends aside, all of the ammonium ions are weak acids (stronger than water; weaker than carboxylic acids), so the amines are moderate bases (stronger than carboxylates, weaker than hydroxide).
Continure to Page 2 to see the impact of conjugation.