The Diamond Lattice
Molecules with all sp3-hybridized atoms can be visualized and drawn using a simple convention. The atom itself (and the bonds to it) are designated by one of two methods:
Draw three lines at 120° angles. One line will either point up or down.
![]()
If the vertical bond line goes up, add a fourth bond below the left-hand bond,
displaced by 30° If the vertical bond line goes down, add the fourth bond above
the right-hand bond, again displaced by 30°.
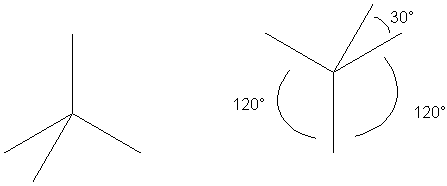
The meaning implied by this convention is that the two bonds separated by 30° are going into and out from the plane of the paper, and the other two bonds are in the plane of the paper.
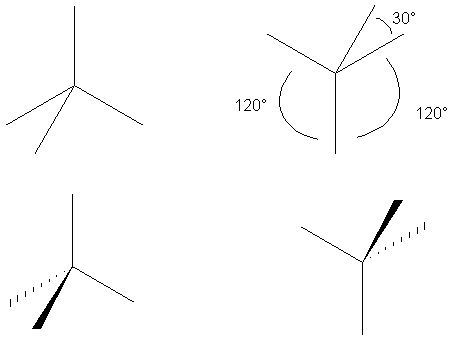
Note that these two are related by 180° rotation.
These two designators fit together in alternating positions to assemble any molecule you
want. Extending it in all three directions makes the arrangement of atoms seen in
the crystal lattice of diamond.
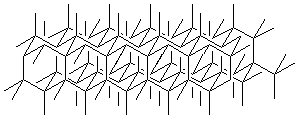
An important feature that helps you in the visualization is that, in this depiction, bonds that are parallel in the diagram are parallel in reality. To test this, make a model of a chair cyclohexane and compare to its diamond lattice representation:
In all of these, the diamond lattice is in grey, and the molecules appear in red.
Rotate by clicking and dragging to get the best view. A good idea is to draw the
molecules, then rotate the view to match the image with your drawing.
First, the diamond lattice:
Cyclohexane
Here, we have both a trans conformation and a cis conformation of butane within the lattice.
Cis and trans-decalins (decahydronapthalene) give you practice with drawing cyclohexane chairs:
Go here if you can't see the structures amid the lattice
Back to Drawing Conventions
Back to CH630 Home Page
