Divalent carbon may have either of two electronic structures. In a singlet, the two unshared electrons are paired in an sp2 orbital, leaving a vacant p orpital. The angle between the substituents is normally 120-140° (steric interactions favor opening the angle somewhat from the ideal 120°).
This can seen in the calculated orbitals for a singlet carbene:
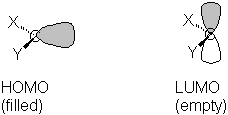
| CH2 Show the LUMO Show the HOMO Measure the H-C-H angle |
|
| CH2_1.pdb | |
| CCl2
Measure the Cl-C-Cl angle |
|
| CCl2_1.pdb | |
| CPh2
Measure the Ph-C-Ph angle |
|
| Ph2C.pdb |
Singlets are favored when there are electron-withdrawing substituents (Cl, O) and when conjugation stabilizes the electron-deficient empty orbital.
In many cases, though, another arrangement is electronically accessible. If the carbon is sp-hybridized, then the ideal geometry is linear. Two p orbitals on the carbon are degenerate (identical energy) and the two electrons remain unpaired, one in each p orbital. (Deviation from linear geometry is common, but the bond angle is always larger than in the singlet state.)
| CH2 Measure the H-C-H angle |
|
| CH2_3.pdb | |
| CCl2
Measure the Cl-C-Cl angle |
|
| Cl2C_3.pdb | |
| CPh2
Measure the Ph-C-Ph angle |
|
| Ph2C_3.pdb |
Triplets are more favored when the substituents are alkyl groups.
In cases where the singlet-triplet gap is low, the Curtin-Hammett principle may apply and reactivity may arise from either or both spin states.
Back to CH630 Home Page
Last updated: 09/21/00
Comments to K. Gable