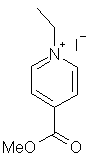
Physical properties of some common organic solvents
| e | m | Y | Z | ET | |
| Water | 84.2 | 2.2 | 3.49 | 94.6 | 63.1 |
| Formamide | 110 | 3.7 | 0.6 | 83.2 | 56.6 |
| Methanol | 38.6 | 3.46 | -1.09 | 83.6 | 55.5 |
| Ethanol/water(80%) | 0 | 84.8 | 53.6 | ||
| Ethanol | 24.3 | 1.69 | -2.03 | 79.6 | 51.9 |
| Isopropyl alcohol | 18.3 | 1.66 | -2.73 | 76.3 | 48.6 |
| Acetonitrile | 36.2 | 3.92 | 71.3 | 46 | |
| Dimethylsulfoxide | 49 | 3.96 | 71.1 | 45 | |
| Sulfolane | 44 | 4.7 | 77.5 | 44 | |
| t-Butanol | 10.9 | 1.66 | -3.26 | 71.3 | 43.9 |
| Dimethylformamide | 36.7 | 3.86 | 68.5 | 43.8 | |
| Acetone | 20.7 | 2.88 | 65.7 | 42.2 | |
| Dichloromethane | 8.9 | 1.6 | 64.2 | 41.1 | |
| Pyridine | 12.3 | 2.19 | 64 | 40.2 | |
| Chloroform | 4.7 | 1.87 | 63.2 | ||
| Hexamethylphosphoramide | 62.8 | 40.9 | |||
| Tetrahydrofuran | 7.32 | 1.63 | 37.4 | ||
| Diethyl ether | 4.34 | 1.15 | 34.6 | ||
| Benzene | 2.28 | 0 | 54 | 34.5 | |
| Toluene | 2.38 | 0.36 | 33.9 | ||
| Carbon tetrachloride | 2.23 | 0 | 32.5 | ||
| Hexane | 1.89 | 0.08 | 30.9 |
Definitions:
e = dielectric constant, a macroscopic property of the solvent as a continuous medium.
m = dipole moment, debyes, a microscopic property of the individual molecule.
Y = log(k(test solvent)/k(80%EtOH/water)) for SN1 reaction of tert-butyl chloride at 25°C. A more polar solvent makes this reaction faster, and Y more positive.
Z = energy of the photochemical excitation of methyl N-ethyl-4-pyridinecarboxylate iodide, kcal/mol, in the particular solvent.
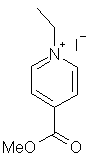
ET = energy of the photochemical excitation of an N-phenoxypryidinium zwitterion, kcal/mol, in the particular solvent.
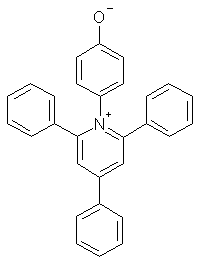
For both Z and ET, the stabilization of the polar reactant vs. the nonpolar excited state leads
to an increase in excitation energy in polar solvents.
Back to CH 362 Home page
02/28/05 ;