 But
increasing efficiency, so that less fuel is used to produce the same
amount of energy, is not a simple engineering task. Greater efficiency
reduces carbon dioxide emissions but increases regulated exhaust emissions
in the form of oxides of nitrogen (NOx), which are a
major source of photochemical smog. If the combustion process is modified
to reduce NOx emissions, these systems then emit large
amounts of particulate matter and carbon monoxide.
But
increasing efficiency, so that less fuel is used to produce the same
amount of energy, is not a simple engineering task. Greater efficiency
reduces carbon dioxide emissions but increases regulated exhaust emissions
in the form of oxides of nitrogen (NOx), which are a
major source of photochemical smog. If the combustion process is modified
to reduce NOx emissions, these systems then emit large
amounts of particulate matter and carbon monoxide. In
spark-ignited engines, which most automobiles today use, another problem
arises when efficiency is increased. With an increase in the compression
ratio comes a tendency for the engine to knock or ping unless the fuel
includes an additive like tetraethyllead or MTBE (methyl tertiary-butyl
ether).
In
spark-ignited engines, which most automobiles today use, another problem
arises when efficiency is increased. With an increase in the compression
ratio comes a tendency for the engine to knock or ping unless the fuel
includes an additive like tetraethyllead or MTBE (methyl tertiary-butyl
ether). This
interplay of efficiency and emissions-and to a lesser extent engine
knock-is at the heart of almost all research today on the automotive
engine and on other practical combustion systems such as gas turbines,
industrial burners, boilers, and so on. Fixing engine knock with a fuel
additive is easy. But recall that leaded fuel was a major air pollutant,
and MTBE is currently polluting groundwater. Reducing exhaust emissions is
equally challenging.
This
interplay of efficiency and emissions-and to a lesser extent engine
knock-is at the heart of almost all research today on the automotive
engine and on other practical combustion systems such as gas turbines,
industrial burners, boilers, and so on. Fixing engine knock with a fuel
additive is easy. But recall that leaded fuel was a major air pollutant,
and MTBE is currently polluting groundwater. Reducing exhaust emissions is
equally challenging. Trial-and-error
research on the combustion engine produced results for many years,
including the first solution to the problem of engine knock. However, by
the middle 1970s, computers had advanced sufficiently for use in detailed,
full-system modeling, a cheaper and more effective way to explore
combustion problems.
Trial-and-error
research on the combustion engine produced results for many years,
including the first solution to the problem of engine knock. However, by
the middle 1970s, computers had advanced sufficiently for use in detailed,
full-system modeling, a cheaper and more effective way to explore
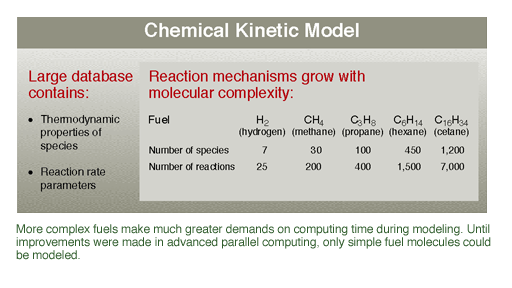
combustion problems. A
challenge for modeling automotive fuels is the enormous variation in their
chemical makeup. On a day-to-day basis, refineries mix and match the
hundreds of components in the fuel they produce to meet certain mandated
specifications. But there are broad tolerances in those specifications,
which largely dictate only that the fuel will make an engine run and that
it meet a particular octane (antiknock) or cetane (diesel-ignition)
rating. This variability is what makes modeling so challenging and so
important. Modeling makes it possible to predict with confidence the
effect of each of the ingredients on the overall fuel mixture.
A
challenge for modeling automotive fuels is the enormous variation in their
chemical makeup. On a day-to-day basis, refineries mix and match the
hundreds of components in the fuel they produce to meet certain mandated
specifications. But there are broad tolerances in those specifications,
which largely dictate only that the fuel will make an engine run and that
it meet a particular octane (antiknock) or cetane (diesel-ignition)
rating. This variability is what makes modeling so challenging and so
important. Modeling makes it possible to predict with confidence the
effect of each of the ingredients on the overall fuel mixture. At
Livermore, modeling of the combustion process was an offshoot of nuclear
weapons research. According to Livermore physicist Charlie Westbrook, an
internationally recognized authority on combustion and combustion
modeling, "Modeling the combustion process really isn't very different
from modeling what happens in a nuclear weapon. Instead of looking at the
reactions of protons and neutrons in a weapon, we started looking at what
was happening to hydrocarbon and oxygen molecules under combustion
conditions."
At
Livermore, modeling of the combustion process was an offshoot of nuclear
weapons research. According to Livermore physicist Charlie Westbrook, an
internationally recognized authority on combustion and combustion
modeling, "Modeling the combustion process really isn't very different
from modeling what happens in a nuclear weapon. Instead of looking at the
reactions of protons and neutrons in a weapon, we started looking at what
was happening to hydrocarbon and oxygen molecules under combustion
conditions." Livermore's
computational capabilities have always been among the best in the world.
Add to that Livermore's multidisciplinary staff and you have a powerful
modeling team. Charlie Westbrook today leads the Chemistry and Chemical
Engineering Division in the Chemistry and Materials Science Directorate,
but for years he was in the trenches leading much of Livermore's
combustion modeling work. Working with computer experts, engineers, and
others, his team produced some revolutionary results. That tradition
continues today in work on a novel engine design, a new method for
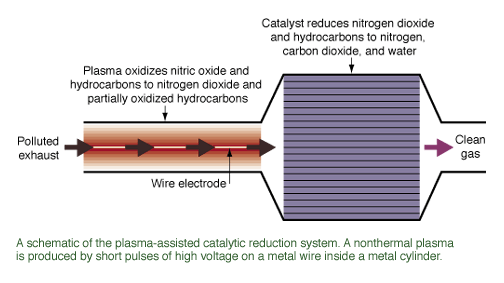
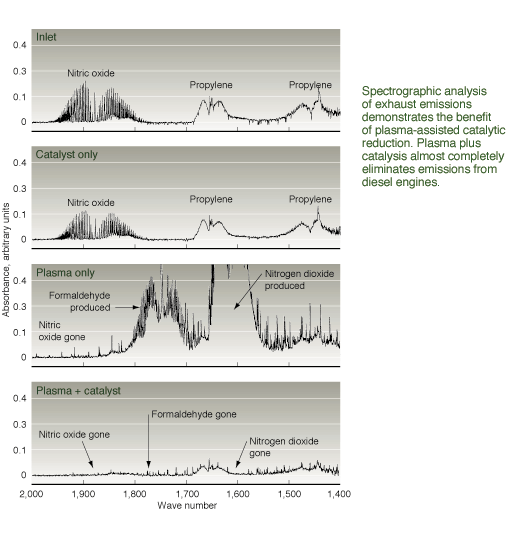
reducing NOx emissions from diesel engines, and reformulated diesel fuels.
Livermore's
computational capabilities have always been among the best in the world.
Add to that Livermore's multidisciplinary staff and you have a powerful
modeling team. Charlie Westbrook today leads the Chemistry and Chemical
Engineering Division in the Chemistry and Materials Science Directorate,
but for years he was in the trenches leading much of Livermore's
combustion modeling work. Working with computer experts, engineers, and
others, his team produced some revolutionary results. That tradition
continues today in work on a novel engine design, a new method for
reducing NOx emissions from diesel engines, and reformulated diesel fuels.
Early Results Livermore
brought its talents to three major cooperative research groups formed in
the middle 1970s by the Department of Energy with Ford Motor Company,
General Motors Corporation, and Unocal. Other participants in these groups
included universities, private industrial firms, and the Los Alamos and
Sandia national laboratories. While working in collaborations that lasted
for almost 20 years, group members won about one-third of the Horning
Memorial Awards given by the Society for Automotive Engineering for the
best paper on engine-fuel relationships presented at the society's annual
meeting.
Livermore
brought its talents to three major cooperative research groups formed in
the middle 1970s by the Department of Energy with Ford Motor Company,
General Motors Corporation, and Unocal. Other participants in these groups
included universities, private industrial firms, and the Los Alamos and
Sandia national laboratories. While working in collaborations that lasted
for almost 20 years, group members won about one-third of the Horning
Memorial Awards given by the Society for Automotive Engineering for the
best paper on engine-fuel relationships presented at the society's annual
meeting. One
major project of the research groups modeled flame quenching at engine
walls. In the cylinder of an automobile engine, a flame ignited by a spark
propagates through an air-fuel mixture and toward the cylinder walls and
piston. Before this project began, engine researchers everywhere were
certain that a major source of unburned hydrocarbon emissions was the
extinguishing of the flame as it approaches the relatively cold walls of
the cylinder. They thought the process left behind a thin layer of
unreacted fuel.
One
major project of the research groups modeled flame quenching at engine
walls. In the cylinder of an automobile engine, a flame ignited by a spark
propagates through an air-fuel mixture and toward the cylinder walls and
piston. Before this project began, engine researchers everywhere were
certain that a major source of unburned hydrocarbon emissions was the
extinguishing of the flame as it approaches the relatively cold walls of
the cylinder. They thought the process left behind a thin layer of
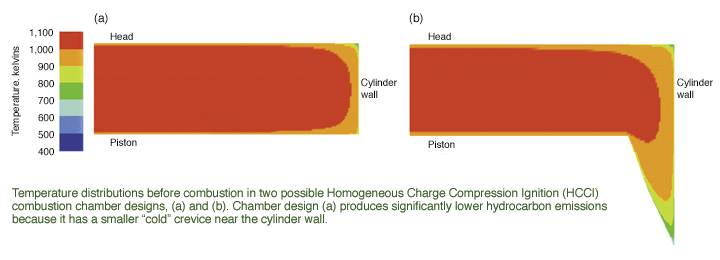
unreacted fuel. Modeling
results seemed to support that traditional view until near the end of the
ignition process, when data indicated that fuel trapped in the cold
boundary layer at the wall begins to diffuse back out toward the
high-temperature region where it is rapidly consumed. Later research
confirmed that unreacted fuel in the piston ring crevices is actually the
primary source of unburned hydrocarbon emissions.
Modeling
results seemed to support that traditional view until near the end of the
ignition process, when data indicated that fuel trapped in the cold
boundary layer at the wall begins to diffuse back out toward the
high-temperature region where it is rapidly consumed. Later research
confirmed that unreacted fuel in the piston ring crevices is actually the
primary source of unburned hydrocarbon emissions. Equally
revealing was a chemical kinetic study of fuel additives for engine knock
in spark ignition engines, for which Westbrook and William Pitz, also of
Livermore, received the Horning Award in 1991.1 Knocking occurs
when the flame from the spark plug does not consume the gases in the
piston chamber fast enough. The remaining "end gases" spontaneously
combust, sending a damaging shock wave across the chamber.
Equally
revealing was a chemical kinetic study of fuel additives for engine knock
in spark ignition engines, for which Westbrook and William Pitz, also of
Livermore, received the Horning Award in 1991.1 Knocking occurs
when the flame from the spark plug does not consume the gases in the
piston chamber fast enough. The remaining "end gases" spontaneously
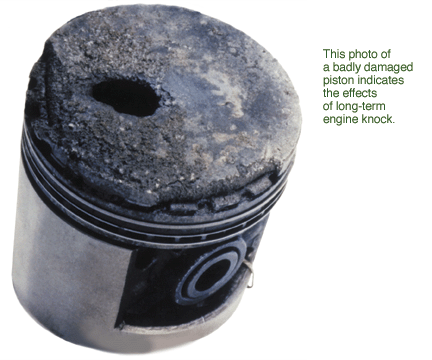
combust, sending a damaging shock wave across the chamber. Engines
operate most efficiently at the highest compression ratios, but that is
precisely where knocking occurs. Engine knock therefore sets an upper
limit to the compression ratio at which a spark-ignited internal
combustion engine can operate. Suppressing knock permits engines to
operate at higher compression ratios and thus to achieve higher fuel
efficiency and lower carbon dioxide emissions.
Engines
operate most efficiently at the highest compression ratios, but that is
precisely where knocking occurs. Engine knock therefore sets an upper
limit to the compression ratio at which a spark-ignited internal
combustion engine can operate. Suppressing knock permits engines to
operate at higher compression ratios and thus to achieve higher fuel
efficiency and lower carbon dioxide emissions. In the
1920s, years before air pollution became a major issue, trial-and-error
research produced an effective solution for engine knock that was used for
decades: the additive tetraethyllead. Since leaded fuel was eliminated in
the 1970s, other additives have been used that work in a variety of ways.
Today, MTBE is the oil refiners' additive of choice for suppressing knock.
But MTBE leaking from underground storage tanks has been found to be
contaminating groundwater (see S&TR, April 1999, pp.
21-23).
In the
1920s, years before air pollution became a major issue, trial-and-error
research produced an effective solution for engine knock that was used for
decades: the additive tetraethyllead. Since leaded fuel was eliminated in
the 1970s, other additives have been used that work in a variety of ways.
Today, MTBE is the oil refiners' additive of choice for suppressing knock.
But MTBE leaking from underground storage tanks has been found to be
contaminating groundwater (see S&TR, April 1999, pp.
21-23). Westbrook
and Pitz's award-winning paper was the culmination of a long-term study of
the fundamental chemical factors that control knocking. Earlier work had
resulted in models for different families of elementary reactions with
varying dependencies on temperature, pressure, and fuel-air
concentrations. Working up from simple to complex molecules, from
single-component fuels to fuel mixtures, the research team simulated the
ignition of fuels with a variety of ignition characteristics. They also
examined an array of proknock and antiknock additives, including MTBE. The
goal of this work was to provide chemical engineers with the ability to
predict the knock behavior of arbitrary mixtures of hydrocarbons and
additives.
Westbrook
and Pitz's award-winning paper was the culmination of a long-term study of
the fundamental chemical factors that control knocking. Earlier work had
resulted in models for different families of elementary reactions with
varying dependencies on temperature, pressure, and fuel-air
concentrations. Working up from simple to complex molecules, from
single-component fuels to fuel mixtures, the research team simulated the
ignition of fuels with a variety of ignition characteristics. They also
examined an array of proknock and antiknock additives, including MTBE. The
goal of this work was to provide chemical engineers with the ability to
predict the knock behavior of arbitrary mixtures of hydrocarbons and
additives.
 Charles K.
Westbrook received a B.S. in physics from Harvey Mudd College and a Ph.D. in
applied science and engineering from the University of California at Davis. He
joined Lawrence Livermore in 1968 in the Physics Directorate, where he served as
division leader of the Computational Physics and Applied Physics Divisions. He
recently became division leader of the Chemistry and Chemical Engineering
Division in the Chemistry and Materials Science Directorate. His honors include
the 1991 Horning Memorial Award from the Society of Automotive Engineers for the
best paper of the year on engine-fuel relationships and the 1992 Thomas Midgley
Award from the American Chemical Society for outstanding contributions in the
field of chemistry related to the automotive industry. Westbrook has authored
approximately 250 refereed publications on combustion, chemical kinetics, and
physics.
Charles K.
Westbrook received a B.S. in physics from Harvey Mudd College and a Ph.D. in
applied science and engineering from the University of California at Davis. He
joined Lawrence Livermore in 1968 in the Physics Directorate, where he served as
division leader of the Computational Physics and Applied Physics Divisions. He
recently became division leader of the Chemistry and Chemical Engineering
Division in the Chemistry and Materials Science Directorate. His honors include
the 1991 Horning Memorial Award from the Society of Automotive Engineers for the
best paper of the year on engine-fuel relationships and the 1992 Thomas Midgley
Award from the American Chemical Society for outstanding contributions in the
field of chemistry related to the automotive industry. Westbrook has authored
approximately 250 refereed publications on combustion, chemical kinetics, and
physics.