
 |
CO2 Capture by Pre-Combustion Decarbonisation of Natural Gas |
![]()
|
by Harry Audus1, Olav Kaarstad2, Geoff
Skinner3 |
The production of electricity from natural gas can be decarbonised by passing the power station’s exhaust gas through a solvent to capture CO2. This route incurs significant cost and energy penalties and there is a need to dispose of degradation products from the amine solvent.
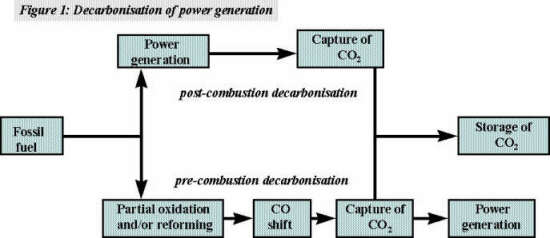
An alternative approach to CO2 capture, i.e. pre-combustion decarbonisation, uses a fossil fuel to make an intermediate hydrogen-rich gas which is then burnt to produce electricity. CO2 capture is an integral part of the process. This route, in the form of an Integrated Gasification Combined Cycle (IGCC) is an option favoured by many for decarbonising the production of electricity from coal, heavy oils, or other carbon intensive fuels.
Pre-combustion decarbonisation is reported on herein as a method of CO2 capture for power generation processes using natural gas. Ways are considered in which hydrogen production combined with isolation of CO2 could be integrated with electricity generation. In all such schemes a hydrogen-rich gas is produced and fired in a gas turbine combined cycle (GTCC). The availability of suitable high-efficiency turbines is likely, but still somewhat uncertain. Two integrated processing schemes are considered in detail (one based on reforming and the other partial oxidation) as methods of producing the hydrogen-rich gas. Energy balance calculations and cost estimates are presented.
The main conclusion is that pre-combustion decarbonisation presents a promising alternative to post-combustion capture of CO2. There is much potential for evolutionary improvements in both pre- and post-combustion decarbonisation technology; both options should be considered as potential means of reducing the emissions of CO2 from power stations and/or turbines fired by natural gas. In the near term, catalytic air-blown partial oxidation could be one of the more attractive options for pre-combustion decarbonisation of natural gas; it appears likely that the treated synthesis gas (roughly 50% H2 and 50% N2) would be preferred over richer hydrogen mixtures as a suitable feed for existing gas turbines.
natural gas, capture of carbon dioxide, decarbonisation, partial oxidation, reforming
Removal of CO2 from the exhaust gases i.e. post-combustion decarbonisation, would probably be the method preferred by most to achieve deep reductions in emissions of greenhouse gases (GHG) to atmosphere if a choice had to be made today. A process based on scrubbing the flue gas with an amine solvent is commercially available but results in considerable cost and energy penalties, e.g. [Barchas].
Pre-combustion decarbonisation is an alternative approach to reducing emissions of GHG from power generation. In this approach, a fossil fuel is used to make a clean gaseous fuel with a minimal carbon content i.e. hydrogen or hydrogen-rich gas mixtures. Removal of CO2 takes place under pressure and is an integral part of the process. The decarbonised fuel is them burnt to produce power with only minimal emissions of CO2 to atmosphere.
In the case of power generation from coal, a pre-combustion decarbonisation option suggested by many e.g. [Goldthorpe] is based on the use of IGCC. In this process, synthesis gas (mainly H2 and CO) produced in a gasifier is shift-converted to CO2 and H2, the CO2 captured with a physical solvent, and the fuel gas burnt in a GTCC. The fuel gas is predominantly hydrogen. It has not always emphasised by the proponents of such schemes that the availability of suitable gas turbines is uncertain (see later). Figure 1 illustrates the pre- and post-combustion decarbonisation approaches.
Partial oxidation of natural gas is an equivalent process to coal gasification; it can be used as a method of producing hydrogen, but most hydrogen is produced by steam-methane reforming (SMR). For economic reasons, partial oxidation is generally restricted to coal, heavy oils, and high molecular weight hydrocarbons which are difficult to steam reform.
Various researchers have raised the possibility of integrating the production of hydrogen and electricity. Originally such ideas were based on production of hydrogen at times of low energy demand. Recently the vision has been of a longer-term future in which renewable energy sources make a major contribution to overall supply of the world’s energy. Perhaps the most extensive work is being done in Japan under the WE-NET(1) programme [Mitsugi].
Conventional steam-reforming processes for the production of hydrogen from natural gas can be readily modified using existing technology to capture most of the CO2 released to atmosphere. We have reported previously on work to assess the implications of decarbonising the production of hydrogen [Audus]. The main conclusions were that a requirement to decarbonise energy production makes hydrogen more competitive as an energy carrier and that such schemes could form a bridge to a longer-term future in which hydrogen is widely used as a clean fuel made from a variety of primary energy sources e.g. renewable, nuclear, fossil. It was also established that hydrogen (with or without decarbonisation) is an expensive feed if it is burnt in a discrete power station to produce electricity.(2) Consequently, ‘carbon-free’ electricity made from decarbonised hydrogen (costing more than twice natural gas per unit of energy) is unlikely to be competitive with electricity produced by post-combustion decarbonisation. However, if the production of hydrogen-rich gas is combined with power generation in an integrated process there are opportunities to reduce the cost and efficiency penalties. This paper discusses the methods by which this synergy can be achieved.
There are at least 4 alternative technologies in common use for the production of the synthesis gas mixtures (H2,CO) required as the first step in pre-combustion decarbonisation (see figure 1). These are SMR, SMR combined with secondary reforming, autothermal reforming, and thermal partial oxidation [Tindall]. Where the presence of nitrogen can be tolerated (or is required such as in ammonia production) air can be used instead of oxygen for the partial oxidation reaction. Previous work by Foster Wheeler has shown that in general, SMR usually seems cheaper than oxygen-blown partial oxidation, but where partial oxidation with air can be used it appears less expensive than SMR.
Following an initial assessment of 5 process configurations (ranging from minimal integration of conventional SMR with combined cycle power generation to highly integrated novel schemes) it was decided to examine three processes in more detail. One of the processes examined in detail, based on steam reforming in pressurised reformers, is not reported here as it did not appear significantly better than the other 2 schemes and would require extensive process development. In all the cases examined it was assumed that CO2 is captured using BASF’s Activated MDEA(3) process, this and a number of equivalent processes are well established. The following describes the 2 processes reported on herein:
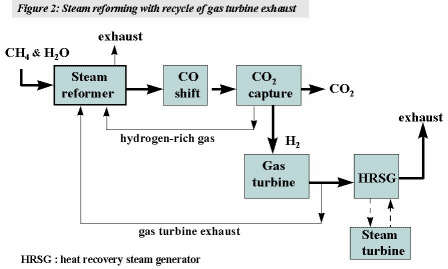
In this process approximately 25% of the gas turbine exhaust gas is recirculated and used as combustion gas in a conventional reformer furnace. Additionally, about 30% of the hydrogen-rich fuel gas produced is used to fire the reformer.(4) This arrangement avoids emissions of CO2 from the reformer furnace. The gas burnt in the turbine combustion chamber is 95% by volume hydrogen. Reformers using gas turbine exhaust gases exist hence, the basic ‘building blocks’ of the processing scheme are proven. (See later for comments on gas turbines.) The process is depicted in figure 2.
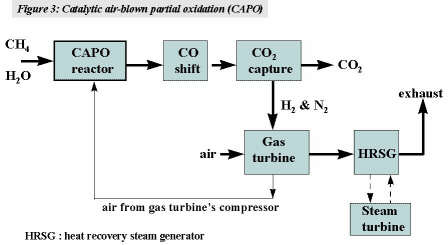
In this process, air and steam are used to partially oxidise natural gas. The air is taken from the gas turbine’s air compressor (at 20 bar). The product gas burnt in the turbine combustion chamber is 53% hydrogen and 43% nitrogen. The process is illustrated in figure 3, and the reactor in figure 4.
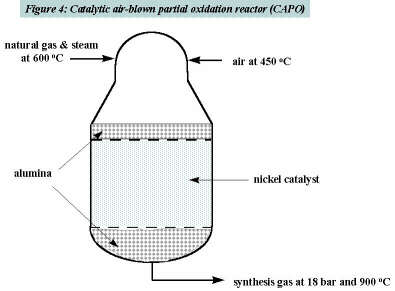
Air, steam, and natural gas are mixed in a conical combustion zone at the top of the refractory lined reactor. In this zone both partial oxidation and steam reforming reactions take place. In the catalyst zone the reactions, including the steam-methane reaction, are brought to near equilibrium. The reactor operates at a pressure dictated by the discharge pressure of the gas turbine’s air compressor. In our calculations the turbine pressure ratio was assumed to be 20:1.
The key process results are presented in table 1. The base-case used as a reference is a state-of-the-art natural gas combined cycle (NGCC). As far as is possible the pre-combustion decarbonisation cases were based on established technology; there is significant potential for improvement. For example, the gas turbines presently under development with a 30:1 pressure ratio might be of advantage in both CO2 capture cases (and the base-case), and it might be attractive to replace part or all of the amine scrubbing process by membrane separation. The minimum level for capture of CO2 was set at a readily attainable 85%; significantly higher levels of capture are possible.
Table 1: Key process results.
|
NGCC (base-case) |
Reforming + GTCC (recycled turbine exhaust) |
Partial oxidation +GTCC (air-blown) | |
|
Efficiency (LHV) |
59% |
48% |
50% |
|
CO2 emissions (gCO2/kWh) |
350 |
60 |
60 |
|
gas turbine fuel (mole%) |
85% CH4 |
95% H2 |
53% H2, 43% N2 |
All the cases considered rely on the availability of a gas turbine which can burn hydrogen-rich mixtures. It is not clear how much development is needed and what barriers need to be overcome to ensure the availability of such turbines.(5) We have discussed the requirements with two turbine manufacturers.(6) It seems clear that the higher the hydrogen concentration the more doubts there are about the availability of suitable turbines. Of the cases examined, this conclusion would seem to favour the air-blown partial oxidation scheme, at least in the short-term, because the fuel is roughly a 50:50 mixture of hydrogen and nitrogen.
The concerns about modification of high efficiency gas turbines to burn hydrogen-rich mixtures appear to be about the low energy density and high flame speed of hydrogen and means of ensuring low NOx emissions. Attempts are being made to clarify the situation. If considerable turbine development is required this could present a serious obstacle to all pre-combustion decarbonisation schemes based on the combustion of hydrogen in gas turbines(7); including those schemes based on coal IGCC which are frequently proposed in the literature. It is however the impression of the authors that the barriers against burning a H2/N2 mixture in large gas turbines may be quite low and only partially of a technical nature.
The extent to which air-blown partial oxidation is an established technology for use with natural gas as a feed is uncertain. It is believed that, apart from secondary reformers in ammonia plants, experience is limited. Foster Wheeler have participated in tests of CAPO in an existing medium-size ammonia plant. Such limited, but encouraging, experience would need to be extended before the process could be adopted with confidence. Other CAPO processes have been suggested at a large scale but, as far as is known, their use in this application has not been demonstrated.
Cost estimates were done for each of the cases assessed; they are considered to be accurate to about +/-30%. The cost estimate did not cover the cost of adapting a normal gas turbine to operate in either of the modes presented here, nor is there any special allowance for meeting specific NOx emission limits. There was some debate about the cost of the base-case NGCC as costs have decreased markedly over the last few years and do not appear to have stabilised. Table 2 illustrates the key cost figures. The cost of natural gas used is 2$/GJ which may be slightly low for most circumstances. A discount rate of 10% was assumed for the financial calculations. The cost of compressing CO2 to 90 bar is included in these figures but the cost of storing CO2 is not. The cost of storing CO2 is very dependent on the distance it has to be delivered; assuming storage deep underground at a site 200km or less from the power station, the additional cost for storage would be about 0.3 c/kWh.
Table 2: Key cost figures (for a standard output of 500MWe).
|
NGCC (base-case) |
Reforming + GTCC (recycled turbine exhaust) |
Partial oxidation + GTCC (air-blown) | |
|
Specific cost (US$/kW) |
630 |
1040 |
940 |
|
Cost of electricity (cents/kWh) |
2.5 |
3.6 |
3.4 |
|
Cost of avoidance in $/t CO2 ($/tC) |
----- |
37 (136) |
27 (99) |
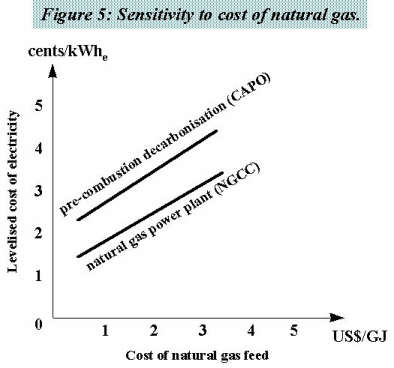
Figure 5 illustrates the sensitivity to the cost of natural gas for the NGCC and the CAPO results.
Typically the cost of post-combustion decarbonisation is reported as adding in the range 1.5 - 2.0 c/kWh to the cost of electricity; for example, see [Leci]. However, improvements to this type of process are being made. [Mimura] has reported on work with sterically hindered amine solvents with a reduced regeneration energy and a reduction in the deposition of heat stable salts, improved column packing, and optimised steam utilisation.
The work reported herein examined the possibility that integrating hydrogen/synthesis gas production with electricity generation might result in a promising approach to reducing emissions of CO2 to atmosphere. Table 3 is a comparison of how the pre-combustion decarbonisation options we examined compare with post-combustion decarbonisation (flue-gas scrubbing). It can be seen from table 3 that pre-combustion decarbonisation is a promising alternative to post-combustion decarbonisation.
As only a limited number of pre-combustion alternatives were examined it is possible that better options might be identified. The slight cost advantage shown in table 3 for pre-combustion decarbonisation has probably been eroded by recent developments in solvent absorption technology. Hence, neither option has a clear advantage and any choice between them at present would probably be project or application specific.
The catalytic air-blown partial oxidation route looks the most promising of the options assessed but there was not a great deal of difference between them; at present it would also seem to be favoured because of limitations in the hydrogen content of fuel for gas turbines.
The availability of turbines suitable for a hydrogen-rich feed is the key to the future development of precombustion decarbonisation processes.
Table 3: A comparison of pre- and post- combustion decarbonisation
|
Flue-gas scrubbing (post-combustion) |
Synthesis gas clean-up (pre-combustion) | |
|
Cost implications (ref. a conventional NGCC) |
Efficiency penalty » 8-10% points Cost of CO2 capture & storage » 1.5 -2.0 c/kWh Cost of avoidance » 50-60$/tCO2 |
Efficiency penalty » 8-10% points Cost of CO2 capture & storage» 1.0 -1.5 c/kWh Cost of avoidance » 30-40$/tCO2 |
|
Development: requirements & possibilities |
Commercially available. Cost reductions and efficiency improvement likely if extensively adopted. |
Requires gas turbine development but this does not appear to be a major problem. CAPO reactor system needs validating. Process design, efficiency and cost improvements are likely. |
|
Acceptability |
Similar to conventional power stations with flue gas treatment. Emissions » 60gCO2/kWh Amine salts degrade to produce a heat stable salt that has to be disposed of. |
Hydrocarbon processing skills required. Emissions » 60gCO2/kWh No major effluents; effect of hydrogen-rich feed on NOx production in turbine is not known. |
|
Applicability |
Widely applicable, possible retro-fit. |
Widely applicable. No retro-fit opportunities. Co-product opportunities e.g H2 and electricity. |
Audus H, Kaarstad O, Kowal M, 11th World Hydrogen Energy Conference, 23-28 June 1996, Stuttgart, Germany, Proceedings, Vol I, pp 525-534
Barchas R, Davis R, Energy Convers. Mgmt Vol. 33, No. 5-8, pp. 333-340, 1992
Gas Turbine World, March/April 1998, pp16-21
Goldthorpe S. H, Cross P.J. I, Davison J. E, Energy Convers. Mgmt Vol. 33, No. 5-8, pp. 459-466, 1992
Leci C. L, Energy Convers. Mgmt Vol. 37, Nos. 6-8, pp. 915-921, 1996
Mimura T, Simayoshi H, Suda T, Iijima M, Mituoka S, Energy Convers. Mgmt Vol. 38, suppl., pp. S57-S62, 1997
Mitsugi C, Harum A, Kenzo F, Int. J. Hydrogen Energy, Vol. 23 No. 3, pp. 159-165, 1998
Pourchot, Molliere, “Heavy duty gas turbines in low calorific value application”, presented at Power-Gen ’98, Milan, Italy, June 9-11th 1998
Tindall B. M, Crews M. A, Hydrocarbon Processing, pp. 75-81, November 1995
This paper contains the views of the authors; it should not be taken as necessarily representing the views of Statoil, Foster Wheeler, or members of the IEA Greenhouse Gas R&D Programme.
(1) International Clean Energy System Technology Utilizing Hydrogen (World
Energy Network).
(2) At a natural gas cost of 2$/GJ and a 10% discount rate
the cost of hydrogen production by steam reforming was 4.3$/GJ; capture and
compression of the CO2 produced increased the cost of hydrogen to
5.6$/GJ.
(3) Methyl-DiEthanolAmine.
(4) The steam reforming reaction is
endothermic; typically requiring about 25-30% of the input energy to the
process.
(5) [Gas Turbine World] reports on turbines being run on refinery
gases; the hydrogen content is limited to a maximum of 40%. On the other hand
[Pouchot] reports on some heavy duty gas turbines running on a refinery gas with
70% hydrogen.
(6) One of which has done practical research aimed at the
combustion of hydrogen in aviation turbines; the other has recently started
practical research looking at burner design for stationary turbines.
(7) The
capital cost of a turbine includes a large element for recovery of the
development cost. Any distinctly novel turbine would be heavily penalised for
this reason.