|
SNOX™ Flue Gas Cleaning Demonstration ProjectEnvironmental Control Devices
|
|
| |
|
Participant Location Plant Capacity/Production Coal Technology |
Additional Team Members | ||||||||
| |||||||||
|
SNOX is a trademark of Haldor Topsoe a/s. | |||||||||
 Project Objective
Project Objective
To demonstrate at an electric power plant using U. S. high-sulfur coals that SNOX™ technology will catalytically remove 95% of SO2 and more than 90% of NOx from flue gas and produce a salable by-product of concentrated sulfuric acid.
Technology/Project Description
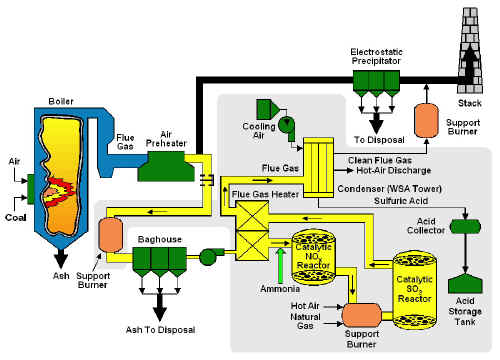
In the SNOX™ process, the stack gas leaving the boiler is cleaned of fly ash in a high-efficiency fabric filter baghouse to minimize the cleaning frequency of the sulfuric acid catalyst in the downstream SO2 converter. The ash-free gas is reheated , and NOx is reacted with small quantities of ammonia in the first of two catalytic reactors where the NOx is converted to harmless nitrogen and water vapor. The SO2 is oxidized to SO3 in a second catalytic converter. The gas then passes through a novel glass-tube condenser that allows SO3 to hydrolyze to concentrated sulfuric acid.
The technology, while using U.S. coals, was designed to remove 95% of the SO2 and more than 90% of the NOx from flue gas and produce a salable sulfuric acid by-product. This was accomplished without using sorbents and without creating waste streams.
The demonstration was conducted at Ohio Edison's Niles Station in Niles, OH. The demonstration unit treated a 35-MWe equivalent slipstream of flue gas from the 108-MWe Unit No. 2 boiler, which burned a 3.4% sulfur Ohio coal. The process steps were virtually the same as for a commercial full-scale plant, and commercial-scale components were installed and operated.
Results Summary
Environmental
-
SO2 removal efficiency was normally in excess of 95% for inlet concentrations averaging about 2,000 ppm.
-
NOx reduction averaged 94% for inlet concentrations of approximately 500-700 ppm.
-
Particulate removal efficiency for the high-efficiency fabric filter baghouse with SNOX™ system was greater than 99%.
-
Sulfuric acid purity exceeded federal specifications for Class I acid.
-
Air toxics testing showed high capture efficiency of most trace elements in the baghouse. A significant portion of the boron and almost all of the mercury escaped to the stack. But selenium and cadmium, normally a problem, were effectively captured in the acid drain, as were organic compounds.
-
Absence of an alkali reagent contributed to having no secondary pollution streams or increases in CO2 emissions.
-
Presence of the SO2 catalyst virtually eliminated CO and hydrocarbon emissions.
Operational
-
Having the SO2 catalyst downstream of the NOx catalyst eliminated ammonia slip and allowed the SCR to function more efficiently.
-
Heat developed in the SNOX™ process was used to enhance thermal efficiency.
Economic
-
Capital cost was estimated at $305/kW for a 500-MWe unit firing 3.2% sulfur coal. The levelized incremental cost was estimated at 6.1 mills/kWh or $219/ton of SO2 removal on a constant dollar basis. Comparable current dollar costs were 7.8 mills/ kWh and $284/ton of SO2 .
Project Summary
Because the SNOX™ process utilized an oxidation catalyst to convert SO2 to SO3 and ultimately to sulfuric acid, no reagent was required for the SO2 removal step. As a result, the process produced no other waste streams.
In order to demonstrate and evaluate the performance of the SNOX™ process, general operating data were collected and parametric tests conducted to characterize the process and equipment. The system has operated for approximately 8,000 hours and produced more than 5,600 tons of commercial-grade sulfuric acid. Many tests for the SNOX™ system were conducted at three loads—75%, 100%, and 110% of design capacity.
Environmental Performance
Particulate emissions from the process were very low (< 1 mg/Nm3) due to the characteristics of the SO2 catalyst and the sulfuric acid condenser (WSA Condenser). The Niles SNOX™ plant was fitted with a baghouse (rather than an ESP) on its inlet. This was not necessary for low particulate emissions, but rather was needed to maintain an acceptable cleaning frequency of the SO2 catalyst. At operating temperature, the SO2 catalyst, because of its sticky surface, retained about 90% of the dust that entered the catalyst vessel. Dust that passed through was subsequently removed in the WSA Condenser, which acted as a condensing particulate removal device (utilizing the dust particulates as nuclei).
Minimal or no increase in CO2 emissions by the process was tied to two features--the lack of a carbonate-based alkali reagent that releases CO2 and the fact that the process recovered additional heat from the flue gas to offset its parasitic energy requirements. This heat recovery, under most design conditions, results in the net heat rate of the boiler being the same or better after addition of the SNOX™ process, and consequently no increase in CO2 generation per unit of power.
With respect to CO and hydrocarbons, the SO2 catalyst acted to virtually eliminate these compounds as well. This aspect also positively affected the interaction of the NOx and SO2 catalysts. Because the SO2 catalyst followed the NOx catalyst, any unreacted ammonia (slip) was oxidized in the SO2 catalyst to nitrogen, water vapor, and a small amount of NOx . As a result, downstream fouling by ammonia compounds was eliminated and the SCR was operated at slightly higher than typical ammonia stoichiometries. These higher stoichiometries allowed smaller SCR catalyst volumes and permitted the attainment of very high reduction efficiencies (>95%).
Sulfur dioxide removal in the SNOX™ process was controlled by the efficiency of the SO2-to-SO3 oxidation, which occurred as the flue gas passes through the oxidation catalyst beds. The efficiency was controlled by two factors--space velocity and bed temperature. Space velocity governed the amount of catalyst necessary at design flue gas flow conditions, and gas and bed temperature had to be high enough to activate the SO2 oxidation, reaction. During the test program, SO2 removal efficiency was normally in excess of 95% for inlet concentrations averaging about 2,000 ppm.
|
|
|
The bottom portion of the SO2 converter catalyst, with the catalyst dust collector hopper mounted on steel rails (center), is shown. |
The SCR portion of the SNOX™ process was able to operate at higher than typical ammonia stoichiometries due to its location ahead of the SO2 catalyst beds. Normal operating stoichiometries for the SCR system were in the range of 1.02-1.05 and system reduction efficiencies averaged 94% with inlet NOx levels of approximately 500-700 ppm.
Sulfuric acid concentration and composition has met or exceeded the requirements of the federal specifications for Class I acid. During the design and construction of the SNOX™ demonstration, arrangements were made with a sulfuric acid supplier to purchase and distribute the acid from the plant. The acid has been sold to the agriculture industry for the production of diammonium phosphate fertilizer and to the steel industry for pickling. Ohio Edison has also used a significant amount in boiler water demineralizer systems throughout its plants.
Air toxic testing conducted at the Niles SNOX™ plant measured the following substances:
-
Five major and 16 trace elements including mercury, chromium, cadmium, lead, selenium, arsenic, beryllium, and nickel
-
Acids and corresponding anions (hydrogen chloride, hydrogen fluoride, chloride, fluoride, phosphate, sulfate)
-
Ammonia and cyanide
-
Elemental carbon
-
Radionuclides
-
Volatile organic compounds
-
Semi-volatile compounds including polynuclear aromatic hydrocarbons
-
Aldehydes
Most trace elements were captured in the baghouse along with the particulate. A significant portion of the boron and almost all of the mercury escaped to the stack. But selenium and cadmium, normally a problem, were effectively captured in the acid drain, as were organic compounds.
Operational Performance
Heat recovery was accomplished by the SNOX™ process. In a commercial configuration, it can be utilized in the thermal cycle of the boiler. The process generated recoverable heat in several ways. All of the reactions that took place with respect to NOx and SO2 removal were exothermic and increased the temperature of the flue gas. This heat plus fuel-fired support heat added in the high-temperature SCR/SO2 catalyst loop was recovered in the WSA Condenser cooling air discharge for use in the furnace as combustion air. Because the WSA Condenser lowered the temperature of the flue gas to about 210° F, compared to approximately 300° F for a typical power plant, additional thermal energy was recovered along with that from the heats of reaction.
Economic Performance
The economic evaluation of the SNOX™ process showed a capital cost of approximately $305/kW for a 500-MWe unit firing 3.2% sulfur coal. The levelized incremental cost was 6.1 mills/kWh on a constant dollar basis and 7.8 mills/kWh on a current dollar basis. The equivalent costs per ton of SO2 removed were $219/ton (constant dollars) and $384 (current dollars).
Commercial Applications
|
|
|
The SNOX™ demonstration at Ohio Edison's Niles Station Unit No. 2 achieved SO2 removal efficiencies exceeding 95% and NOx reduction effectiveness averaging 94%. Ohio Edison is retaining the SNOX™ technology as part of its environmental control system. |
The SNOX™ technology is applicable to all electric power plants and industrial/institutional boilers firing coal, oil, or gas. The high removal efficiency for NOx and SO2 makes the process attractive in many applications. Elimination of additional solid waste (except ash) enhances the marketability in urban and other areas where solid waste disposal is a significant problem.
The host utility, Ohio Edison, is retaining the SNOX™ technology as a permanent part of the pollution control system at Niles Station to help Ohio Edison meet its overall SO2/NOx reduction goals.
Commercial SNOX™ plants also are operating in Denmark and Sicily. In Denmark, a 305-MWe plant has operated since August 1991. The boiler at this plant burns coals from various suppliers around the world, including the United States; the coals contain 0.5-3.0% sulfur. The plant in Sicily, operating since March 1991, has a capacity of about 30 MWe and fires petroleum coke.
Contacts
Paul Yosick, Project Manager
ABB Environmental Systems
1409
Center Port Boulevard
Knoxville, TN 37932
(423) 693-7550
(423)
694-5213 (fax)
paul.yosick@usapc.mail.abb.com
Lawrence Saroff, DOE/HQ, (301) 903-9483 lawrence.saroff@hq.doe.gov
James U. Watts, NETL, (412) 386-5991 mailto:watts@fetc.doe.gov