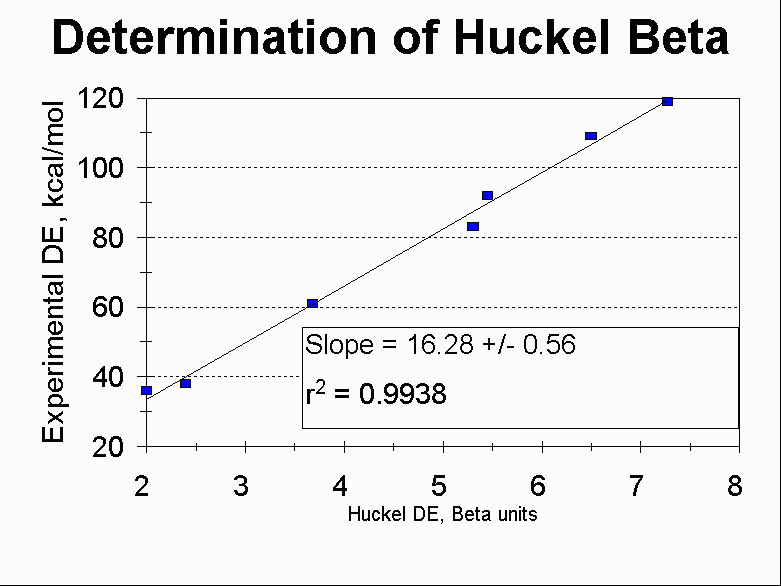

Compounds used in this comparison:
| Structure | p Energy (Hückel) |
Aromatic Stabilization Energy | Experimental Resonance Stabilization |
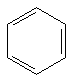 |
8 b | 2 b | 36 kcal/mol |
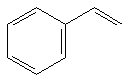 |
10.42 b | 2.42 b | 38 kcal/mol |
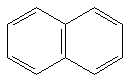 |
13.68 b | 3.68 b | 61 kcal/mol |
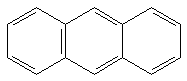 |
19.31 b | 5.31 b | 83.5 kcal/mol |
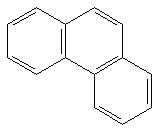 |
19.45 b | 5.45 b | 92 kcal/mol |
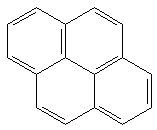 |
22.51 b | 6.50 b | 109 kcal/mol |
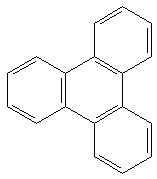 |
25.27 b | 7.27 b | 119 kcal/mol |
(Experimental resonance energies are taken as the difference between the heat of hydrogenation and the heat of hydrogenation for a number of ethylene molecules equivalent to the number of formal double bonds.)
Back the CH 637 Home