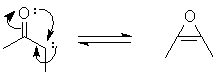
Answers to Carbene/Free Radical Problems
12.6. a. Fragment peroxide; abstract H from aldehyde.
b. H-atom abstraction from the benzylic position; addition to C=C; H-atom abstraction in a chain mechanism.
c. Cleave the peroxide (misprint in text); abstract H from the tin hydride; add tin radical to NO2 group. Cleavage of N-C gives tertiary benzyl radical and Bu3SnNO2; continue the chain with H-atom transfer.
d. Chromium (II) is a one-electron reductant. Add electron to the N-Cl bond, then lose chloride to form a nitrogen-centered radical. Add to the double bond, then abstract chlorine atom to continue the chain.
e. One-electron transfer and loss of NO2- leads to 2-(4-pyridyl)propyl radicals, which dimerize.
f. Reduction of the alcohol to an alkoxy radical; fragmentation to acetophenone and a 2-propyl radical. The propyl radical adds to the acetonitrile; the intermediate pulls an H atom from the alcohol and gets hydrolyzed (on workup) to the amide.
g. Ring opening to a trimethylenemethane diradical.
h. Generate the thiophenyl radical; add to the terminal C=C; add to O2; cyclize; add to O2 (again); H-atom transfer from thiophenol.
12.7. Abstract H atom and lose CO. Reversibly form the cyclopropylcarbinyl radical to make the two methylene groups equivalent. In the second case, a concentrated solution makes H-atom transfer competitive with ring closure.
Additional problems.
a. Form dichlorocarbene; cyclopropanate; open cyclopropane with expulsion of Cl-; loss of proton.
b. Form neopentyl carbene; migrate methyl group.
c. Form the carbene, and instead of a "normal" Wolff rearrangement, perform a cycloc rearrangement to a ketene. Addition of MeOH to the C=C forms the product.
d. You need to make a symmetric intermediate: form the carbene, then (reversibly) close to an oxirene:
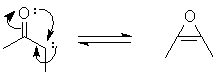
A Wolff rearrangement to the product is slower.
Back to Homework page
Back to CH 630 Home page
Last updated: 10/09/03