Answers to Homework Set 3
A.
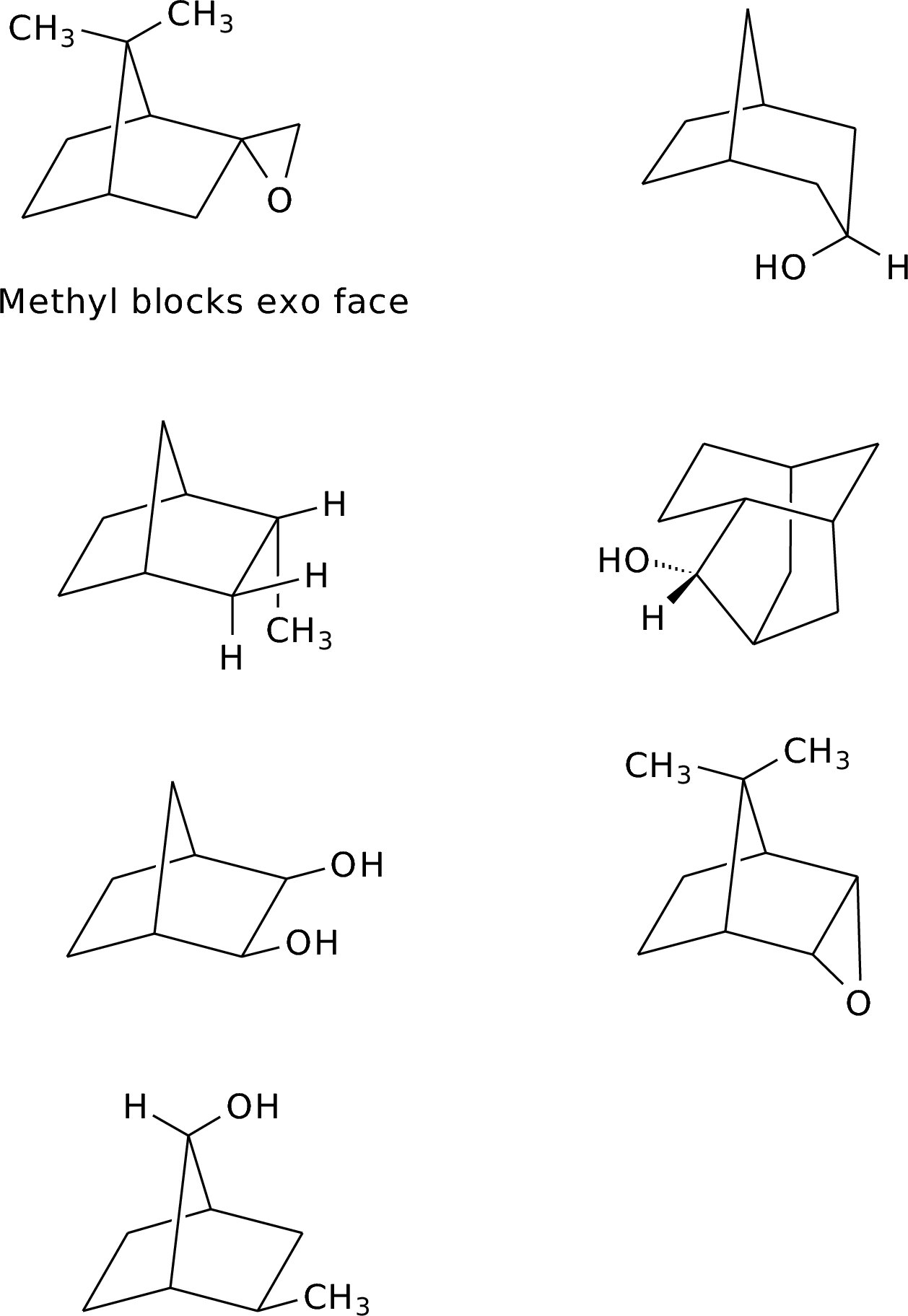
2.7.All are governed by steric control of access to the reaction site.
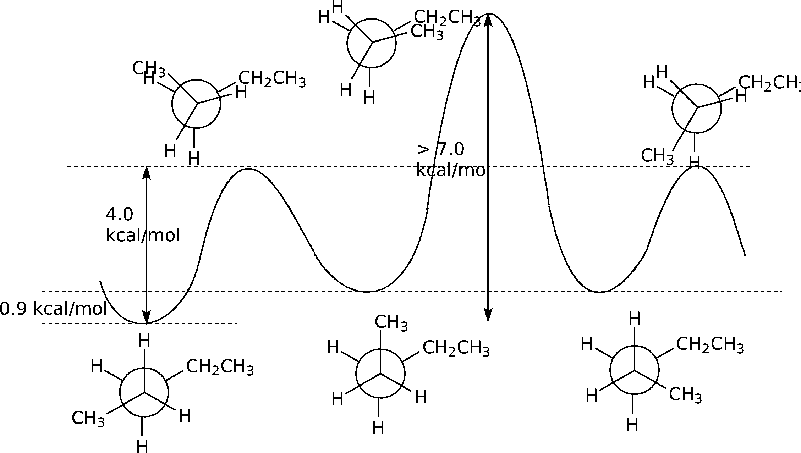
2.19. First, some basic stereochemistry. Ignoring for a moment any restriction on the biphenyl (more on that in a moment), one expects the two isomers to each have some symmetry that would make the two methyl groups (and the methines) equivalent: the cis would be meso, and the trans would have a C2 axis of rotation.
The temperature dependence indicates that each isomer at low temperature gets locked into a conformation where the methyls are different from each other. For isomer A, this occurs at a relatively high temperature (at or below room temperature) so there is a barrier to form a symmetrical conformation that is quite high; for isomer B that barrier is lower.
Furthermore, the unequal intensity for isomer B at low temperature indicates not simply freezing the molecule into a single structure having distinct methyls, but more likely is freezing into two different conformations, each with some symmetry (static or average).
Looking at models, we see the following:
| 3_20_cis.pdb | 3_20_trans1.pdb | 3_20_trans2.pdb |
The twist about the biaryl forces the cis form to always have two unique methyls (unless the positions average out). However, in the trans form, the two methyls are always equivalent, but there are two forms that can equilibrate! A = cis, B = trans. The positional inversion in cis A requires rotation about the biaryl linkage (high barrier), while the interconversion of the two trans forms of B is rotation about the CH(Me)-CH(Me) bond.
1.

2. It's easiest to adapt the original Michael addition, adjusting the size of the ring and the location of the carbonyl to suit.
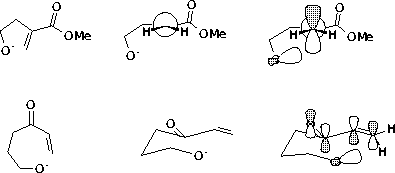
3. The α form experiences anomeric stabilization by donation of electrons from the ring oxygen. This lengthens the C-Br bond, and makes the reactant look more like the transition state for C-Br cleavage. Thus, the energy of the transition state for the α compound will be closer to the starting material (lower) than will the energy of the transition state for ionization of the β isomer, and α will react faster than the β.
Back to Homework page
Last updated: 10/09/03