Answers for Problem Set 1
1.2. The
ability to maintain aromaticity (4n + 2
electrons in a continuous loop; n=0,1,2,...) is the important
observation.
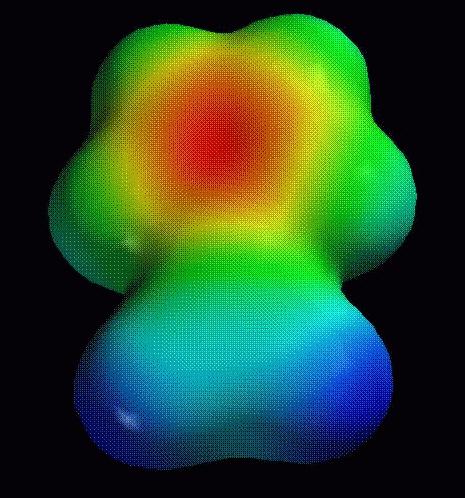
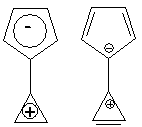
a. Place the electrons from the central double bond in the 5-member
ring. To confirm the
validity of this approach, look at the electrostatic potential map
(red=negative,
blue=positive; calculation at the PM3 semiempirical level):


b. Conjugate the
electron pair of the amine with the nitro group.
c. Draw the resonance structures for each in which a heteroatom lone
pair participates in
pi bonding.
1.3. All
can be rationalized on the basis of resonance.
When resonance in the reactant does not help you, draw resonance
structures for all
possible protonated products; this is
usually more informative than
examination of reactants.
a. On the nitrogen.
b. On oxygen.
c. C-2! (Count resonance structures)
d. The nitrogen in the ring (compare aniline vs. pyridine).
1.4. All of these deal with resonance delocalization of the
amide lone pair into the π bond of the carbonyl.
a. Both resonance and MO pictures can be modeled on an allyl anion.
b. The N=C-O form is more polar (+)N=C-O(-) than the N-C=O form;
solvent interactions will thus make it more important (by stabilizing
it).
c. The bicyclic molecule cannot delocalize the lone pair (the four
substituents on the putative N=C bond are not coplanar). Thus, the
carbonyl is more electron-deficient, and more reactive to nucleophiles.
2.1. a.
Diastereomers
b. Enantiomers
c. Enantiomers
d. Diastereomers (both are meso!)
e. Enantiomers
f. Enantiomers
2.2. a. S
b. R
c. R
d. R
e. S
f. 3 stereocenters!C-1: R. C-2: R. C-4: S.
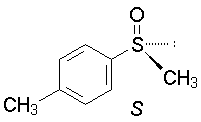
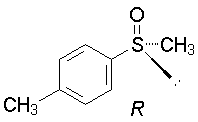
g.The sulfur has a lone pair; atomic number = 0, so the given structure is R.
 |  |
2.26.a.
Chiral
b. Chiral
c. Chiral
d. Achiral (3 planes)
e. Achiral
f. Chiral
g. Chiral (Hexahelicene;
there is a spiral twist.)
h. Chiral
i. Chiral
j. Chiral
k. Chiral
l. Chiral
m. Chiral; see the two forms:
| 2_14ma.mol | 2_14mb.mol |
Supplemental problems.
B.1.
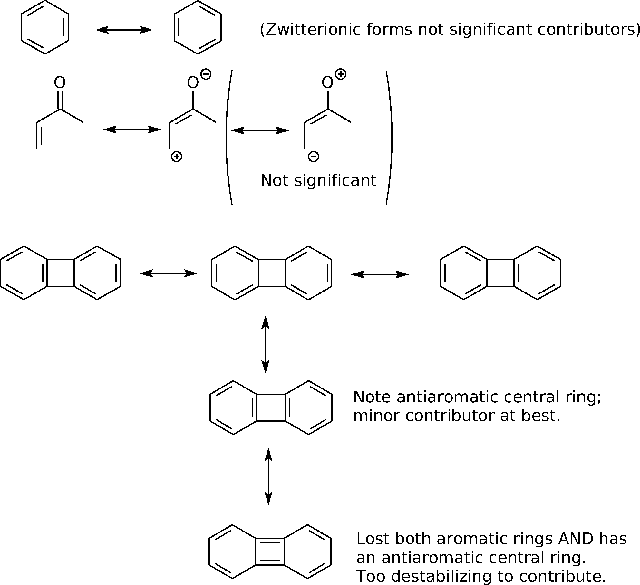
B2.
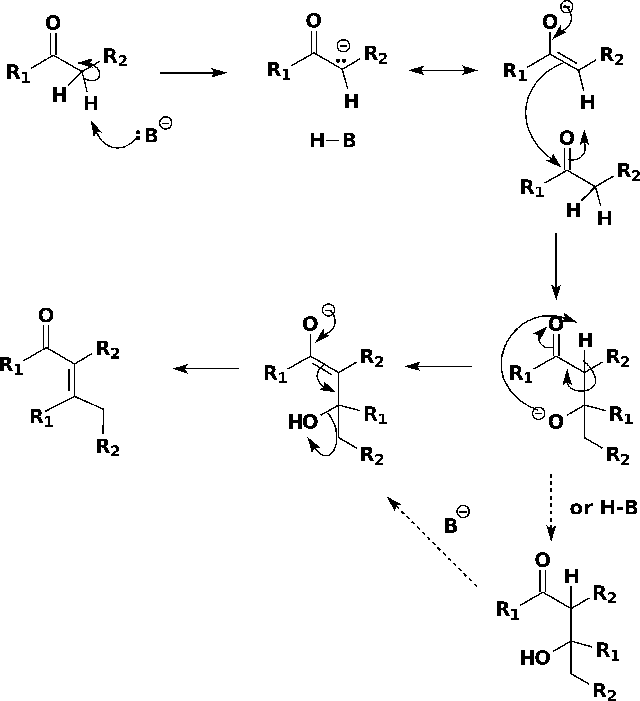
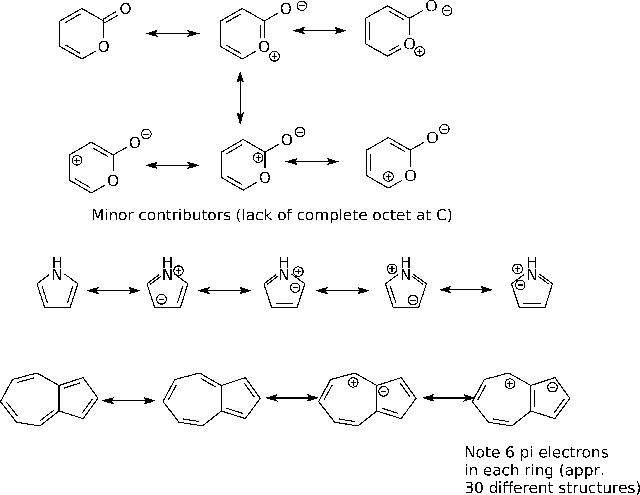
B3.
| a.
cis-1,2-aminomethylcyclohexane (NOT bis-(aminomethyl)cyclohexane!) |
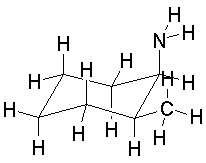 |
| b.
A gauche pentane Others are possible |
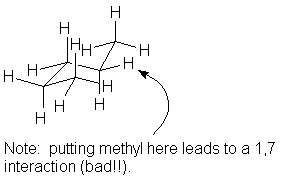 |
| c. 1-Methylnorbornene (1-methylbicyclo[2.2.1]hept-2-ene) | 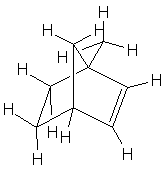 |
| d. Z-bicyclo-[4.3.1]-dec-1-ene (Z indicates the geometry of the double bond; 1-ene makes it a bridgehead alkene with the double bond in the largest ring.) | 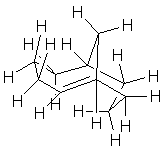 |
| e. trans-decalin (trans-bicyclo[4.4.0]decane) | 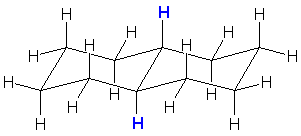 |
| f. cis-decalin | 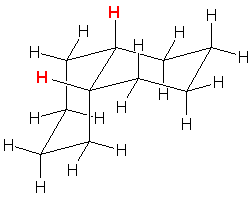 |
| g. trans-4-tert-butylcyclohexanol
Note that the diequatorial form limits steric clashes; the diaxial form has significant H-H interactions with the other axial hydrogens (1,7 interactions). | 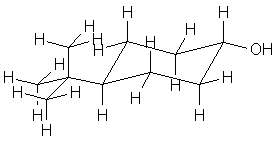 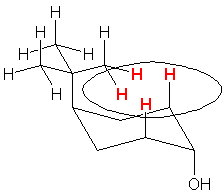 |
| Isobutane (show all hydrogens) | 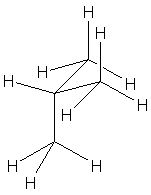 |