Homework Set 1
Due Friday, October 10 in class.
A. Problems from Carey & Sundberg:
1.1, 1.2, 1.4, 2.1, 2.2, 2.26.
B. Additional problems.
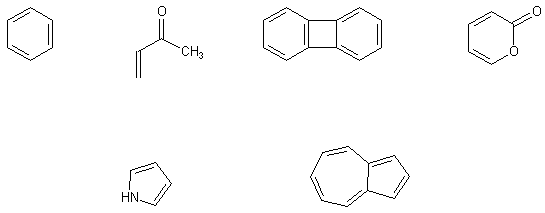
B1. Draw the canonical forms representing all major resonance contributors to the following structures. Where there are large numbers (>5) of essentially identical structures, you may verbally express what they will look like. Be sure to include zwitterionic forms when and if they are expected to contribute significantly to the structure and reactivity of the compound.

B2. The aldol condensation is an example of a reaction that proceeds via a multistep mechanism. The structures below are all involved in the accepted mechanism. Assuming that B-H is a stronger Brønsted acid than the ketone, make proper use of electron-pushing arrows, equilibrium arrows, and irreversible reaction arrows to create a mechanism for this reaction. You should feel free to explicitly show hydrogens or other atoms/molecules that may be involved.
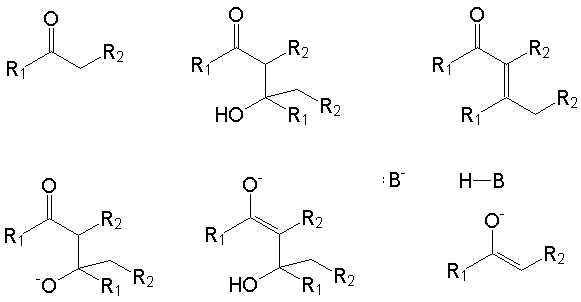
B3.
Draw 3-dimensional perspective representations of the following structures. Where possible, make use of the diamond lattice vertex convention. (Show the correct location of all hydrogens.)
a. cis-1,2-aminomethylcyclohexane
b. A gauche conformer of pentane
c. 1-Methylnorbornene (1-methylbicyclo[2.2.1]hept-2-ene)
d. Z-bicyclo-[4.3.1]-dec-1-ene
e. trans-decalin (trans-bicyclo[4.4.0]decane)
f. cis-decalin
g. trans-4-tert-butylcyclohexanol
h. Isobutane
Back to Homework Main
Page
Back to CH630 Home Page