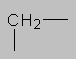
All chemical shifts are expressed in relative terms. The shift, , is expressed in parts per million (ppm) versus some reference compound. For 1H and 13C, this reference compound is normally tetramethylsilane (TMS), (CH3)4Si. A compound having a chemical shift of 1.00 ppm would show a peak 60 Hz downfield of TMS if run on a 60 MHz spectrometer, or 400 Hz downfield of TMS on a 400 MHz spectrometer. Chemical shift is thus independent of the instrument's field strength.
For 1H spectra of most organic compounds, chemical shifts can range from 0-10 ppm. The spectrum is divided into several general regions, although there is a large amount of overlap.
0-0.8 ppm Rarely seen; typical of cyclopropanes, silanes, other organometallic species.
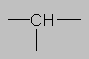
0.8-1.5 ppm Alkane region. Hydrogen attached to aliphatic carbon with no other electronegative substituent. Here and elsewhere, the more substituted the carbon, the further downfield the proton will be (CH>CH2>CH3 in chemical shift).
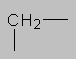 |
 |
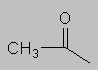
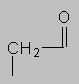
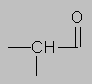
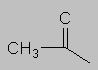
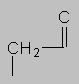
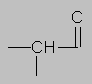
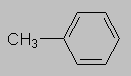
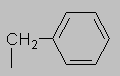
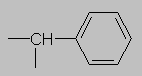
1.5-2.5 ppm Carbonyl region. Protons attached to carbons next to a C=O, C=C or phenyl ring come here. Each of these groups induces a slight polarization of the C-H bond, decreasing electron density and deshielding the proton.
 |
 |
 |
 |
 |
 |
 |
 |
 |
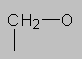
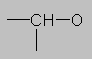
3.0-4.5 ppm Ether region. (Also alcohols and esters have the CH-O group.) Protons on carbon next to a highly electronegative element such as oxygen (or halogens) come here. If there are more than one electronegative substituents, the proton may come even further downfield (note CHCl3 at 7.27 ppm).
 |
 |
|
 |
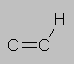
5.0-7.0 ppm Double bond region. Hydrogens directly attached to C=C double bonds come here.
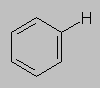
7.0-8.0 ppm Aromatic region. The electronic properties of an aromatic ring lead to a phenomenon called anisotropy (AAHN-eye-SOH-tro-pee) that deshields aromatic protons relative to other protons on sp2 carbon.
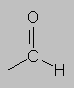
9-10 ppm Aldehyde region. Carbonyls also exhibit anisotropy, and on top of significant electronegativity of the C=O group, this leads to large deshielding.
Variable peaks:
-OH Alcohols can literally appear at any region of the spectrum. The nature of this peak is strongly affected by factors which affect H+ exchange: pH, concentration, temperature, solvent. Generally phenols and more acidic alcohols are further downfield, but this is not necessarily a reliable predictor.
-NHR, -NH2Like alcohols, these have exchangeable protons. However, there is more uniformity in that most amines and amides show broad resonances in the 2-3 ppm range.
-CO2H Also exchangeable, like alcohols, but normally highly deshielded (>11 ppm)
Some examples:
Caution! These files are quite large, and require the Chime 2.0 plug-in. This section is under test (2/23/98).
2-pentanol
Cinnamic acid
Cyclohexanone
Thanks to the Pacific Lutheran University FTNMR FID library for these spectra.
Good tables of NMR chemical shifts for other functional groups may be found in:
Return to NMR page
Return to CH362 Home Page
Comments to K. Gable