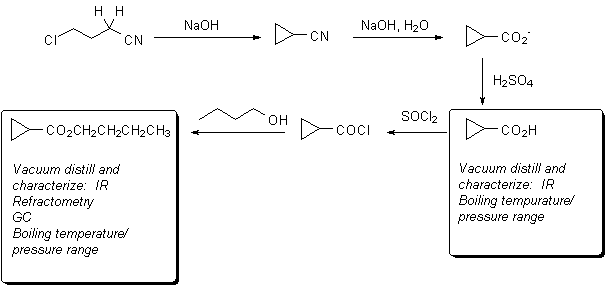
Preparation of n-Butyl Cyclopropanecarboxylate
The chemistry you will use is outlined below:
A nice reference concerning this class of cyclization: https://dx.doi.org/
10.1016/S0040-4020(01)01134-6. (On-campus or VPN/proxy access required)
You should be aware of several safety issues.
- The chlorobutyronitrile is of the same chemical class as iodomethane, so keep it in the
fume hood, try not to spill it, and use gloves throughout the experiment.
- Powdered sodium hydroxide is very caustic; it is also extremely hygroscopic (it absorbs
water from the atmosphere). Take care not to spill it, and transfer it to your
reaction flask as quickly as possible.
- The organic compounds used in this experiment are volatile and have disagreeable odors,
so avoid keeping open containers of any of these outside the fume hood.
- Addition of water to hydrolyze the nitrile causes a large amount of heat as the excess
NaOH dissolves. Add water in 5-mL increments at first to avoid excessive heat
buildup.
Nitrile hydrolysis mechanism
Review of mechanism for acid chloride formation
Grading:
50 points (out of 150 total) are possible for "Results." You will be graded on yield, purity
and characterization you relate in Report I.
- You should calculate the yield of pure carboxylic acid from chlorobutyronitrile.
- You should calculate the yield of butylcyclopropanecarboxylate from
cyclopropanecarboxylic acid.
- You should then calculate the overall yield, which is the product of the two
individual yields.
- Your refractive index for the ester should match the literature value (1.4260 ± 0.0003
at 25°C); an acceptable temperature-corrected value will be within ±.0005 of this.
- You should take IR spectra of both the carboxylic acid and the ester; you need to
identify any peaks in the functional group region, and establish that the fingerprint
region matches that of a reference spectrum.
- You should demonstrate with gas chromatography that the purity of the liquid ester is
>99%. If the butanol peak is >1% the area of the ester peak, you must
redistill. Be sure you have confirmed the retention time of each compound, and that
the GC needle is not contaminated!
- You will need to report the boiling range and pressure for each compound; this, like
melting points of solids, is a characteristic physical property.

