
This experiment will take advantage of the ability to measure relative concentrations from an NMR spectrum.
Acetylacetone (2,4-pentanedione) exists in two isomeric forms, shown below. The Keto form is on the left, and the Enol form is on the right. These two interconvert with each other, but the process is slow enough that an NMR spectrum will show signals from each separate isomer.

|
|
If you are especially observant, you will see tthe possibility of a slightly different type of isomerism. This process is fast on the NMR time scale, and we see signals that imply the symmetry of the average species, shown on the bottom.

Use the NMR Chemical Shift Tables to predict where the peaks will come for each form. How many signals will you see from the keto form? How many from the enol form?
There are several possibilities for how to calculate the equilibrium constant. Let's define this as
Ideally, since these are isomers, we could add up all the integrals for peaks coming from the enol form, and divide this by the total integral for all peaks for the keto form. However, the hydroxyl proton is usually far downfield, and often difficult to integrate accurately. We could apply a correction factor if we ignore this peak, but there is a simpler method.
Both forms have six protons per molecule in methyl groups. Therefore, if we compare the integrals for the methyl singlets, we will directly obtain a measure of the equilibrium constant.!
Alternatively, if we compare the CH peak in the enol form with the CH2 peak in the keto form, we would have to multiply by 2, but this also gives us an independent measure of Keq.
When you are finished, submit your results and look at the overall results from others in the class.
If you use methanol-d4 as a solvent, you have a final complication. The hydroxyl deuterium of the solvent exchanges with the hydroxyl proton of the sample, leading to deuterium washing into the acetylacetone:
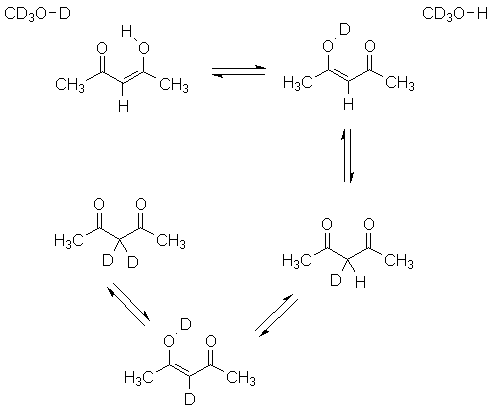
How do you think this will affect how the peak integrals reflect the amount of compound present?
Results submission form
Back to CH362 Home Page
Comments to K. Gable