The reference point (0 ppm) is also the chemical shift of carbon in tetramethylsilane, (CH3)4Si.
Here is a table of typical 13C chemical shifts:
| Chemical Environment of
the Carbon |
200+ |
180 |
160 |
140 |
120 |
100 |
80 |
60 |
40 |
20 |
0 |
| Alkane CH-CR3 |
10-50 |
||||||||||
| Allylic, Benzylic, ketone
=C-CH, Ph-CH, CH-C=O |
40-55 |
||||||||||
| Alkyne C=C-H |
70-110 |
||||||||||
| Alkyl halide CH-X |
55-80 |
||||||||||
| Ether/alcohol/ester CH-O |
60-80 Acetals: 90-100 |
||||||||||
| Nitriles, RC=N |
110-120 |
||||||||||
| Alkene =C-H |
120-160 |
||||||||||
| Aromatic Ph-H |
125-170 |
||||||||||
| Aldehyde, Ketone RC(=O)-H |
>200 |
||||||||||
| Carboxylic Acid RCO2H
and derivatives (esters, acid chlorides, amides, anhydrides) |
165-190 |
||||||||||
| 200+ |
180 |
160 |
140 |
120 |
100 |
80 |
60 |
40 |
20 |
0 |
|
Some examples
Spectrum |
Structure & Notes |
| tBuOH.jdx | 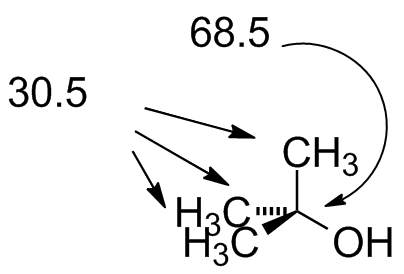 Note the 1:1:1 triplet at 77 ppm: this is CDCl3 solvent. The carbon couples to the deuterium (spin = 1) and creates this pattern. |
|
trimethylpentene.jdx |
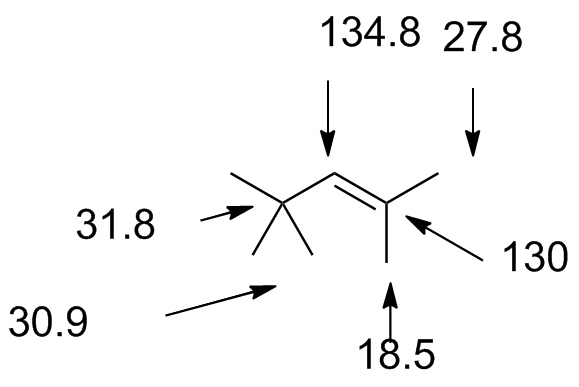 |
|
ochlorobenzoic.jdx |
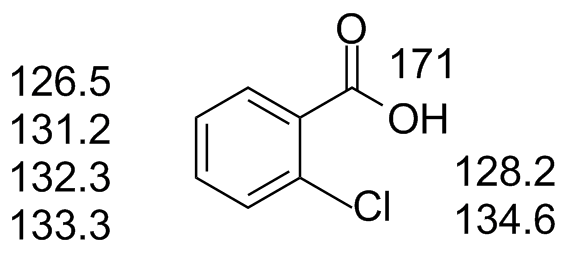 It is possible to predict which carbon is which based on additive substituent effects on each carbon. Those of you in CH 362 are learning how to do that. |