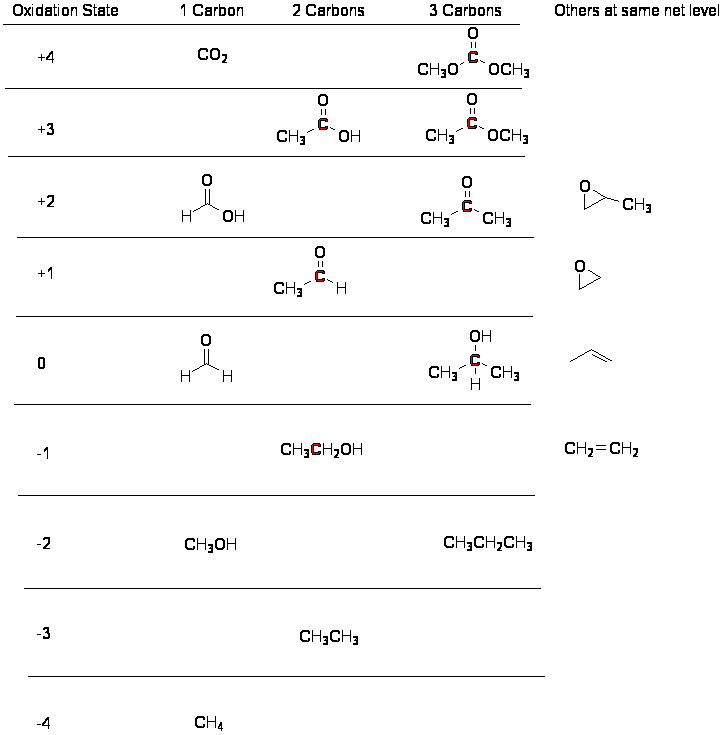Reduction
and oxidation is more complex in organic compounds than in inorganic
compounds because the assignment of formal oxidation states is confused
by the covalent nature of the bonds: the electrons are really shared
and don't "belong" to one or the other atom in the same way that they
do in ionic compounds. However, a few general principles do apply:
- Adding oxygen is usually an oxidation.
- Adding hydrogen is usually a reduction.
- Heteroatoms of any sort (N, P, S, halogens) = oxygen, for
redox purposes.
- Addition or loss of water (or NH3, or
HCl, etc.) causes no change in oxidation state for the
molecule. Essentially, this leads to a formal oxidation at
one site and a reduction at a second site.
The following table lists some formal assignments of
oxidation state for the red carbon atoms. Pay less attention to the
oxidation state than to the relationships along the oxidation/reduction
direction.
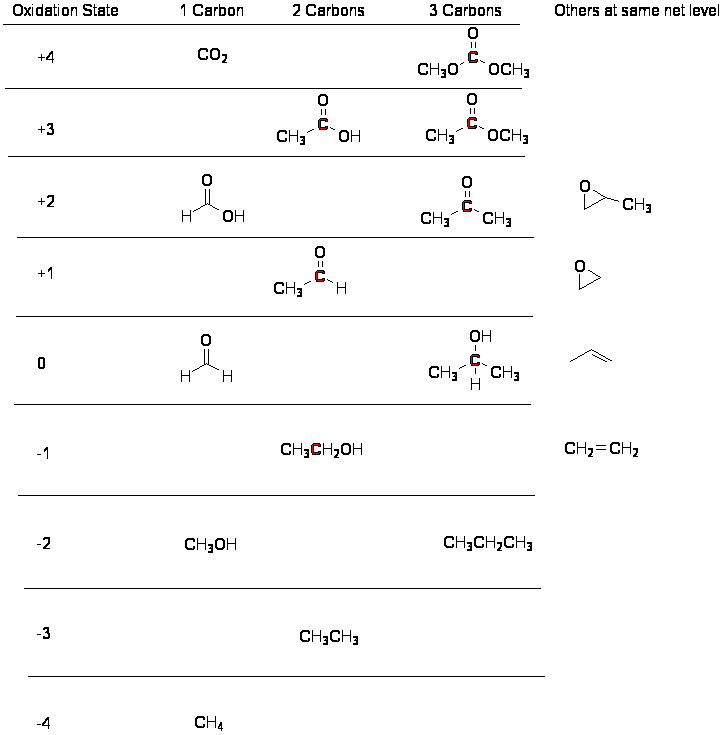
|