Sharpless-Katsuki Epoxidation: An Enantioselective Catalytic Reaction
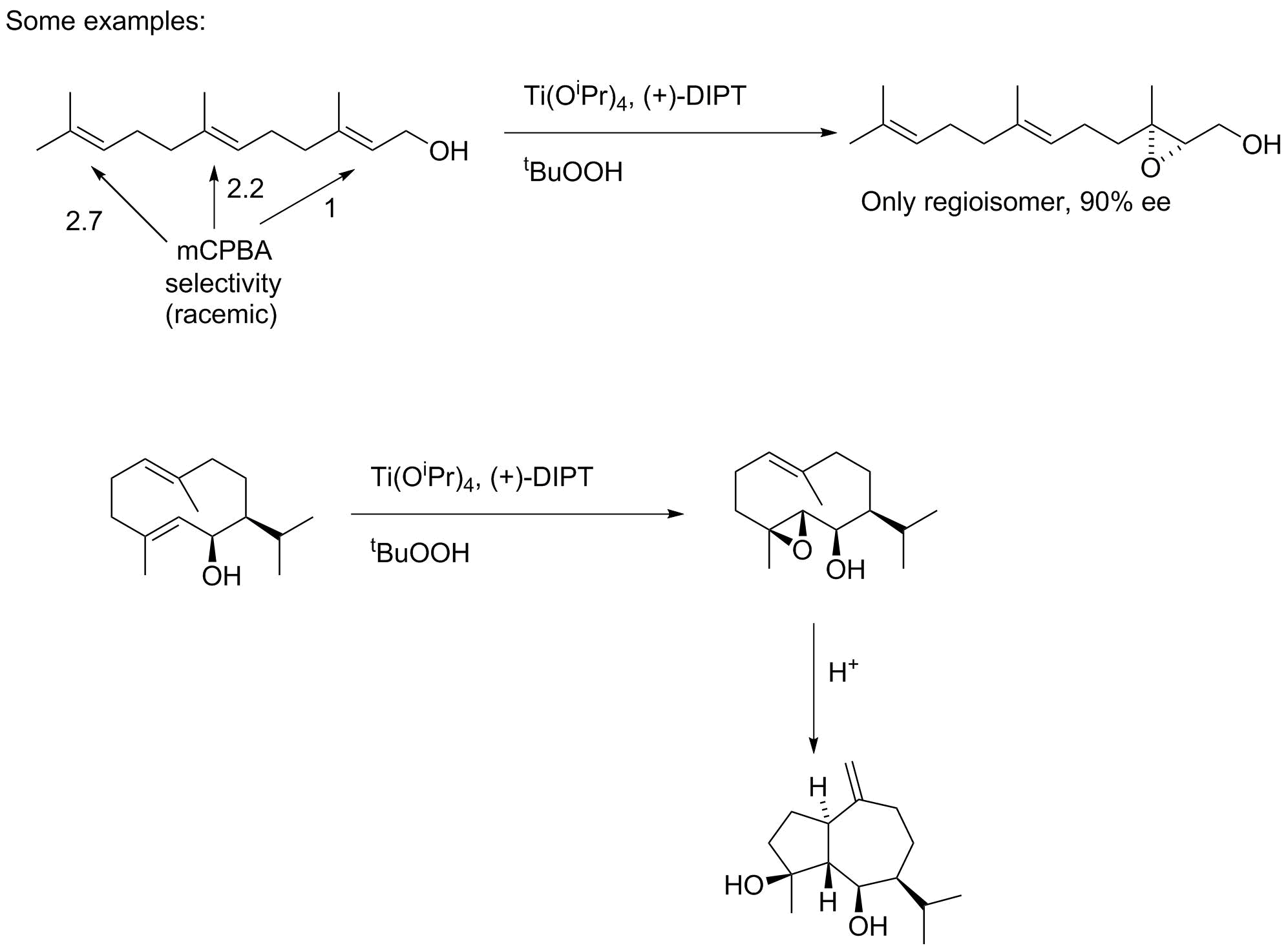
An important discovery in the 1980s was that allylic alcohols could be epoxidized in a regular way to predictably give one enantiomer over another in high yield and selectivities approaching 99%. K. Barry Sharpless won the Nobel Prize in 2001 in part for his role in this discovery.
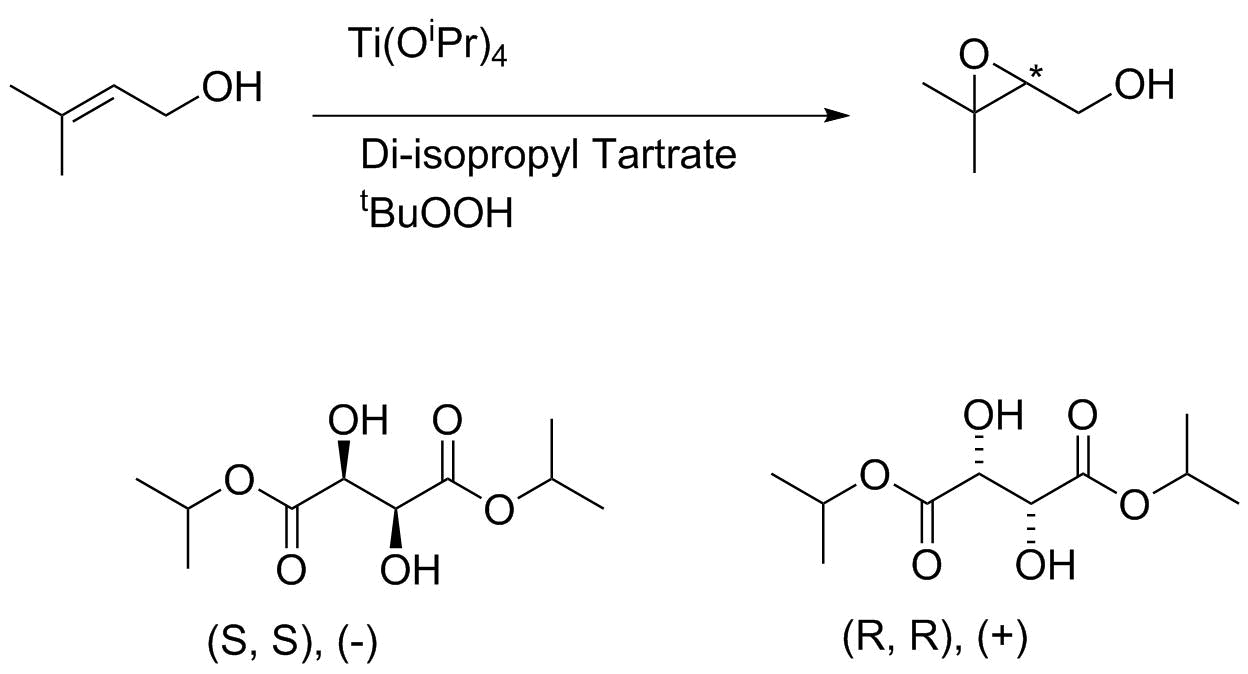
The key is use of a titanium catalyst, that self-organizes to the structure shown below. The alcohol group binds to the titanium, as does the oxidant (tBuOOH). The chiral environment is created by use of one of the two enantiomers of diisopropyl tartrate; both are readily available.
Show the Peroxide Ligand
Show the chiral pocket
Show all three, with hydrogens.
Reload the catalyst structure
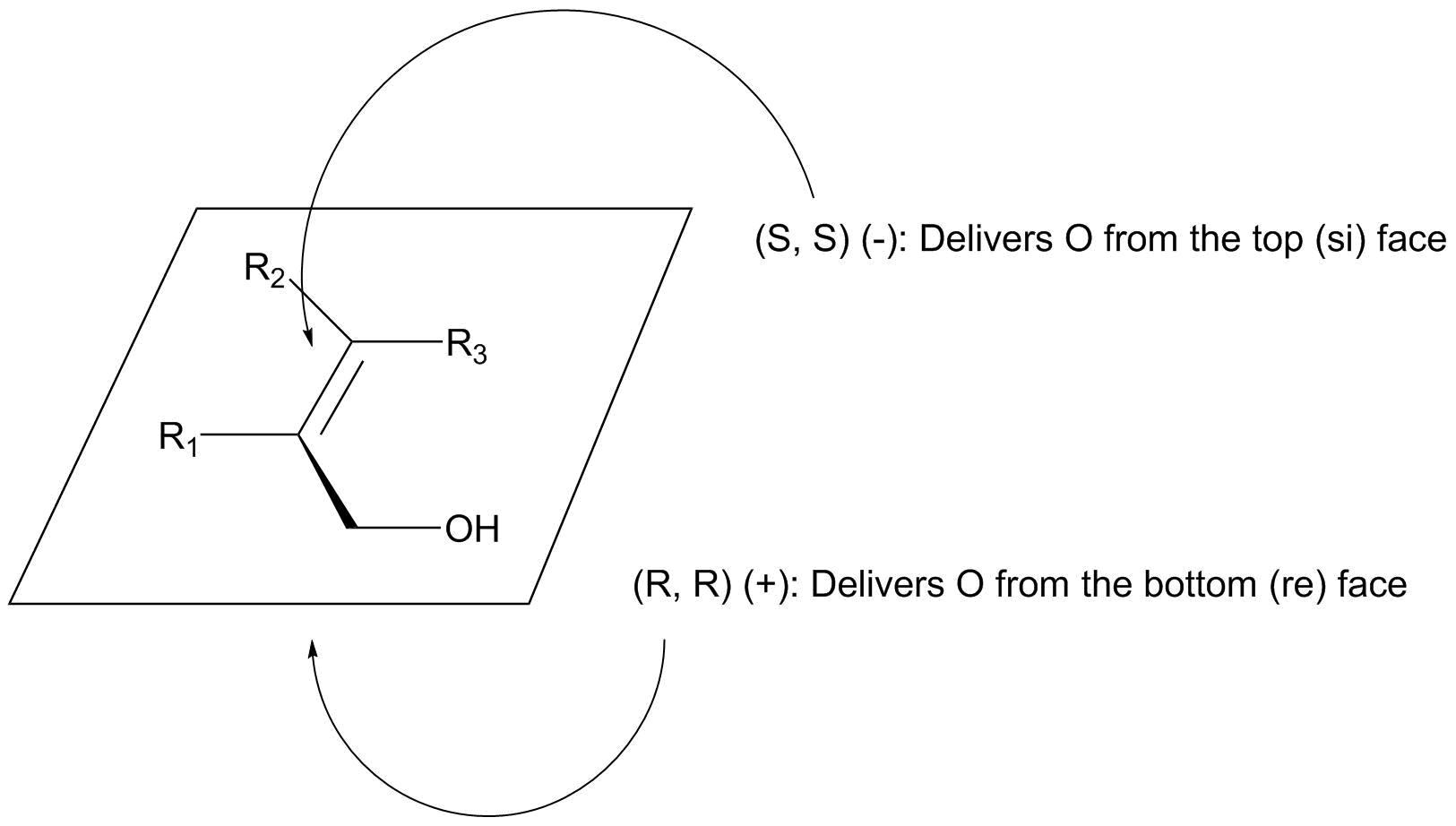
Notice that the esters on the tartrate are pointed towards to of the four quadrants. This creates a chiral "windmill" environment. The alkene π bond can really only approach the Ti-bound oxygen (which gets transfered) using one of its two faces. This results in a predictive mnemonic: place the allyl alcohol in a plane with the OH group pointing towards you and to the right. The (+) tartrate will result in a high ee epoxidation from the bottom (re) face; the (-) enantiomer will give predominant attack from the top (si) face.