Citronellol
|
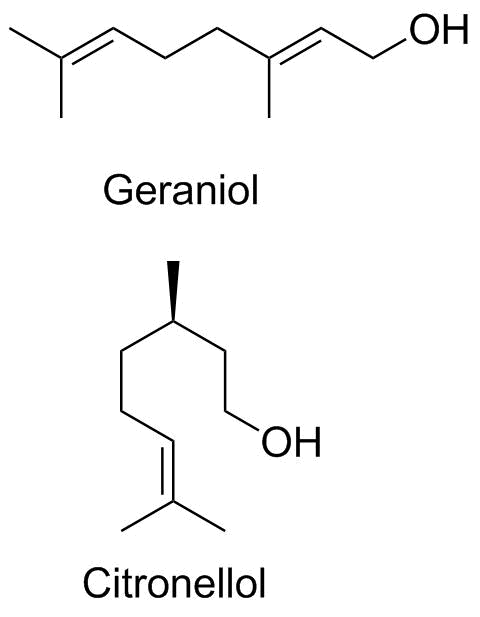
Geraniol Citronellol |
Geraniol and citronellol are both relatively small molecules that contribute to the odor of roses, geraniums andother flowers. Geraniol is also a key intermediate in the synthesis of terpenes, a large class of secondary metabolites found in both plants and animals. |
|
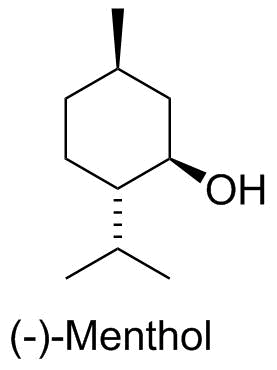
Menthol |
Menthol is one of the major components of a variety of mint plants. We obviously find it in a variety of flavoring agents, usually as the crude mixture in peppermint oil (isolated from steam distillation from the leaves of the mint plant). |
|
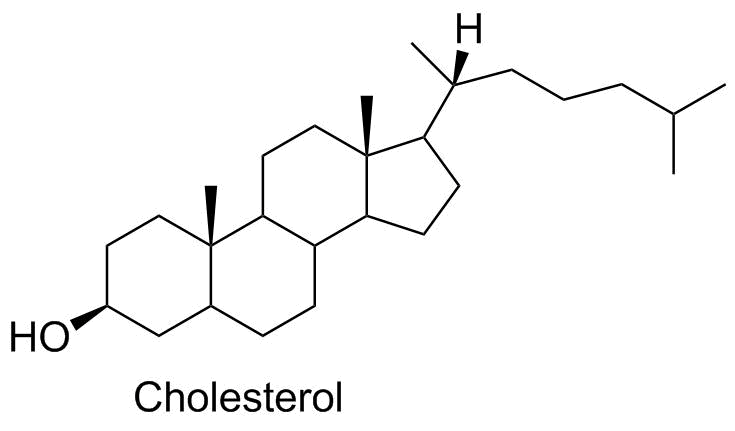
Cholesterol |
Cholesterol (the molecule) is a critical piece of the cell membrane; it interacts with phospholipids to help orient the side chains in the lipid bilayer. Outside the cell, it can trigger other less desirable chemical outcomes, and excess serum cholesterol is often a marker for a range of cardiovascular diseases. |
|
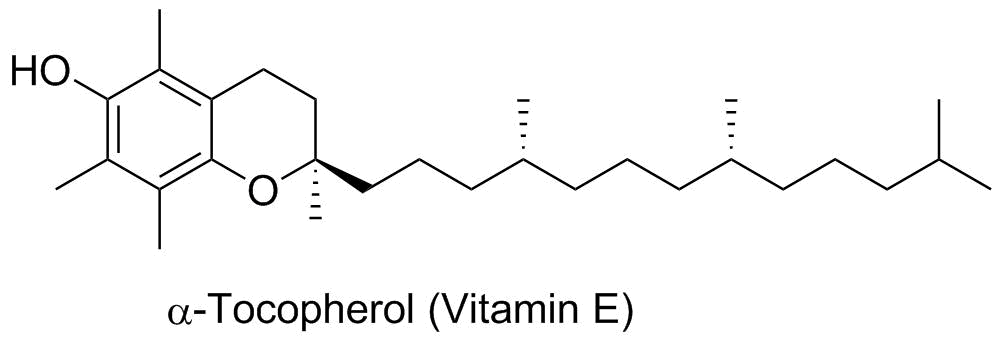
Tocopherol: Vitamin E |
Vitamins are often used as "coenzymes" and can be thought of as "reagents" that interact with enzymes to promote specific chemical functions. Vitamin E is thought to be primarily an antioxidant (the phenol group is similar to BHA and BHT food additives), but it is also involved in a variety of other functions such as kinase protein regulation, gene expression and neurologic functions. This is an example of an aromatic alcohol, or a phenol. |