Hydrogen bonding is a weak (appr. 5 kcal/mol) attraction between a hydrogen in a polarized X-H bond and a lone pair on another atom:
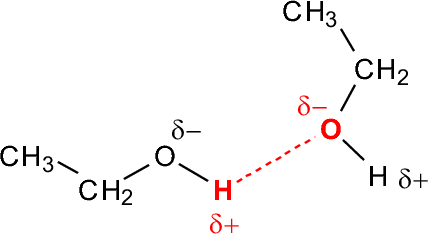
In pure alcohols, this will form a network of extended interactions:
The consequence of these interactions is seen largely in the boiling
point of alcohols, which are much higher than those of alkanes or
ethers with similar molecular weights.
Another consequence is that alcohols have much higher solubilities in
water than alkanes or ethers, and many smaller alcohols are completely
miscible (infinitely soluble) with water. It's pretty easy to imagine
how H2O would substitute for ethanol in the above diagram.