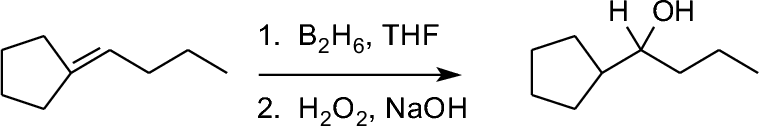One of the new skills you want to develop is to figure out how a particular compound can be made. This involves "thinking backward": analyzing what key bonds are present in the target molecule, and recognizing what reactions you know that will make one (or more) of those. Usually there will be more than one solution, and your task at that point is to select the one that is likely to give the highest yield with the least effort; issues like hazards and waste also factor in.
Retrosynthesis of alcohols is fairly straightforward. The oxygen-bearing carbon (I'll call this the carbinol carbon) has four bonds to it, and any of the four might be appropriate targets for a synthesis. Let's look at one example, a secondary alcohol:
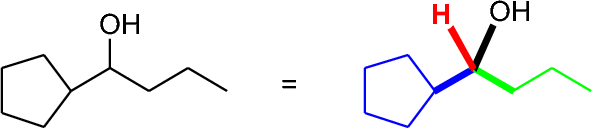
The four bonds we might make are the following (color-coded):
- The cyclopentyl-to carbinol bond (blue)
- The propyl-to carbinol bond (green)
- The hydrogen-to carbinol bond (red)
- The C-O bond (black)
Each of the first three can be formed by adding a nucleophile to a C=O double bond, then protonating. The nucleophile will differ based on the choice we make, as will the carbonyl compound; our final selection might then be based on how easy it would be to buy or make either reactant.
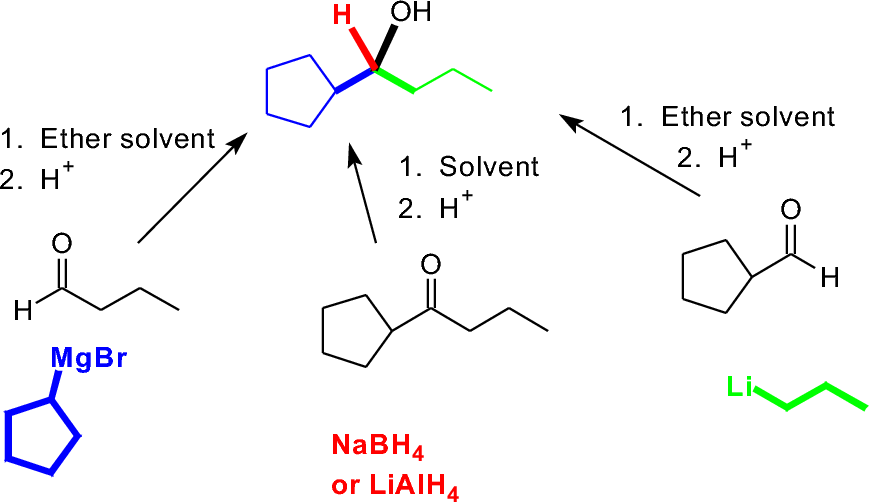
Obviously, a primary alcohol is easier to analyze this way (either an aldehyde + hydride, or an organometallic reagent + formaldyhyde). How might a teritary alcohol be different?
The fourth option, formation of the C-O bond could take place in several ways. Direct oxidation of a C-H bond might do it, but we haven't seen much of this type of reaction (and it is, in fact highly unselective). In some cases, biological enzymes can accomplish this.
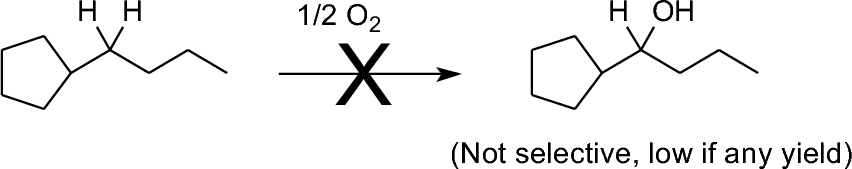
You have seen two different reactions that results in formation of a C-O single bond. Alkene hydration (or oxymercuration/reduction) is one. We might place the C=C bond in either of two places, but in each case we have a selectivity problem:
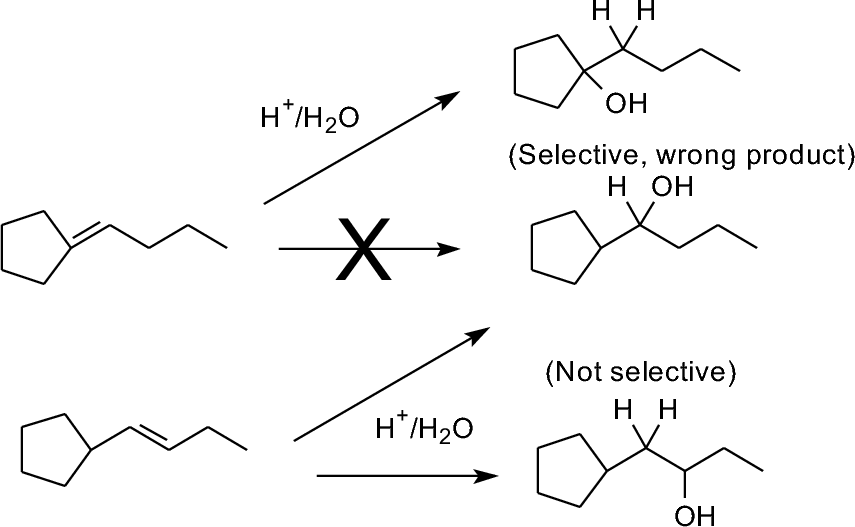
The second, though, hydroboration/oxidation, can work if we choose the better of the two alkenes: