Alcohols are broadly classed into four types, based on the number of C-C bonds to the oxygen-bearing carbon:
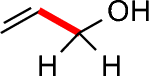
- Primary alcohols have one C-C bond (methanol is also usually considered primary on the basis of its similar reactivity).
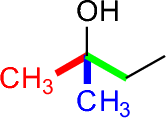
- Secondary alcohols have two C-C bonds to the alcohol carbon
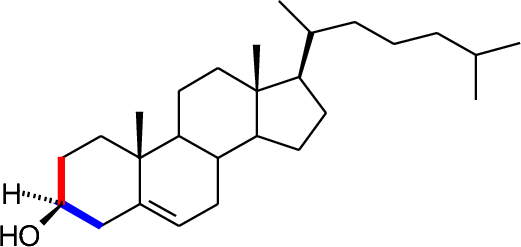
- Tertiary alcohols have three C-C bonds to the alcohol carbon.
- In phenols, the O-H group is on a carbon that is part of an aromatic ring.
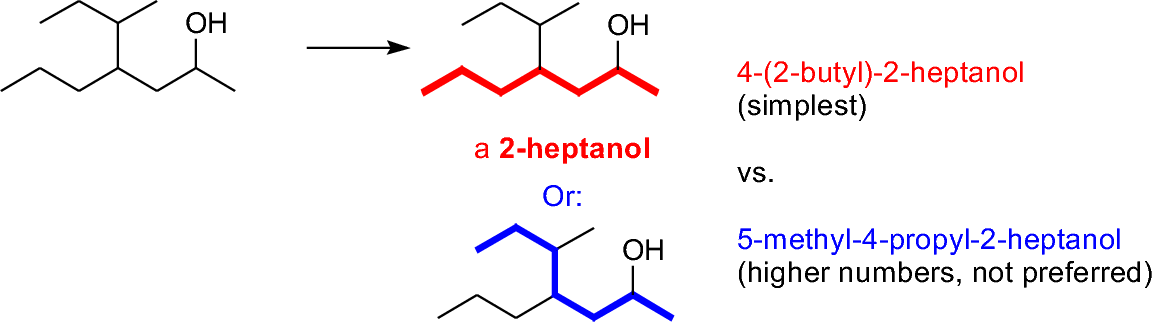
Normally, an alcohol is the major functional group that defines systematic nomenclature. The name of an alcohol ends in -ol. To find the root name, locate the longest carbon chain containing the alcohol, and assign (where there is a choice) the location of the alcohol with the lower of the possible numbers. Then locate any other branches or substituents.
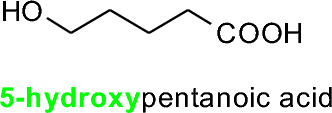
If a higher-priority group exists (like the carboxylic acid shown), the OH group is named as a hydroxy substituent.
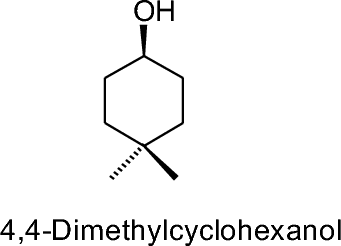
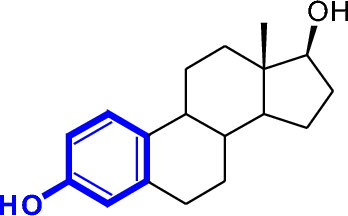
In cyclic alcohols, the carbon bearing the OH is understood to be carbon 1.