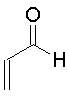
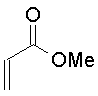
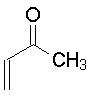
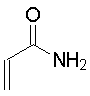
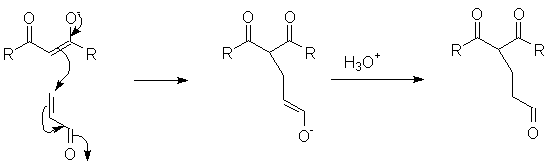
The Michael addition is simply the conjugate addition of an enolate nucleophile to an α, β-unsaturated carbonyl compound. Protonation of the initial intermediate gives a chain-extended product. Any sufficiently electron-withdrawing group on the acceptor double bond will allow this reaction to proceed.
We can generalize the behavior of different nucleophiles as follows:
- "Hard" nucleophiles have their lone pair and charge very localized and are not polarizable. Examples are hydroxide, fluoride, and alkyllithium compounds.
- "Soft" nucleophiles have their lone pair either quite delocalized, or in a large orbital. Examples are iodide, thiolates, cuprates and enolates. Amines and alkoxides also behave like this.
Like acidity and basicity, "hardness" and "softness" are relative measures. The utility of thinking this way is that a hard nucleophile will react fastest with a hard electrophile, and a soft nucleophile with a soft electrophile.
In the context of the Michael addition, the carbonyl carbon is a "harder" electrophile, and the β-position is a "softer" electrophile.
Take a look at a series of Michael acceptors. In these plots, the LUMO is mapped onto the electron density surface, so that the blue regions are the areas of best electron-accepting ability, while the red regions are not electron accepting.
Load LUMO density maps
| Propenal
|
|
| Methyl propenoate
|
|
| 2-Buten-3-one (Methyl vinyl ketone)
|
|
| Propenoamide
|
|
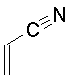
| Propenitrile (acrylonitrile)
|
|
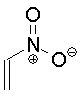
| Nitroethene
|