All carbonyl groups next to a hydrogen-bearing carbon are capable of isomerizing to a different form called an enol. The name is derived from the fact that the isomer has a double bond (ene) and an alcohol (ol) group. The reaction is a net migration of a hydrogen from carbon to oxygen. Because there is a change in nuclear position, we use the equilibrium arrow between them, not the double-headed resonance arrow.
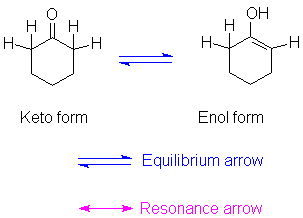
The equilibrium normally lies far to the left, favoring the keto form. Some structural features can encourage formation of an enol.
This reaction is seen for C=O groups and not for C=C groups, largely because of the polarization of the C=O double bond. Although it does occur slowly under neutral conditions, it is catalyzed effectively by both acids and bases.
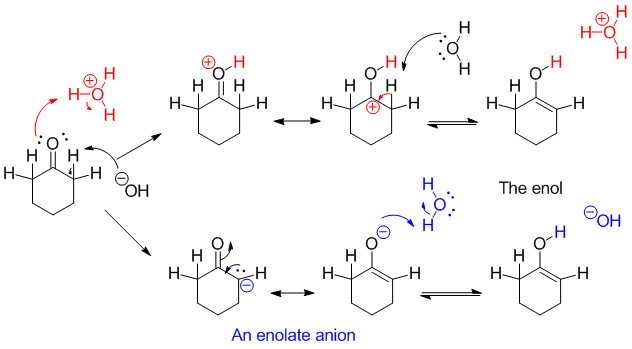
Each of these catalytic processes may be explained by a careful look at the carbonyl group.
- The oxygen has two lone pairs, either of which may be used to bond to an acid (Lewis or Bronsted). Doing so creates a resonance-stabilized carbocation; loss of thegenerates a C=C π bond (like in E1 eliminations), and leaves the neutral enol.
- The positive character of the carbonyl carbon makes the α-proton more acidic than it would be in a hydrocarbon. The pKa of cyclohexanone (in THF or DMSO) is about 25; that for the allylic position in cyclohexene is about 43. That means ketones are 1018 better acids than allylic protons in alkenes (in general).
In each case, the entire process is reversible.