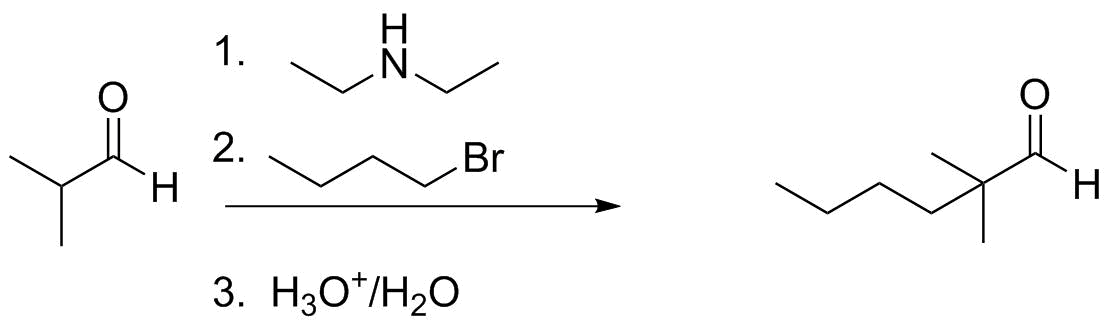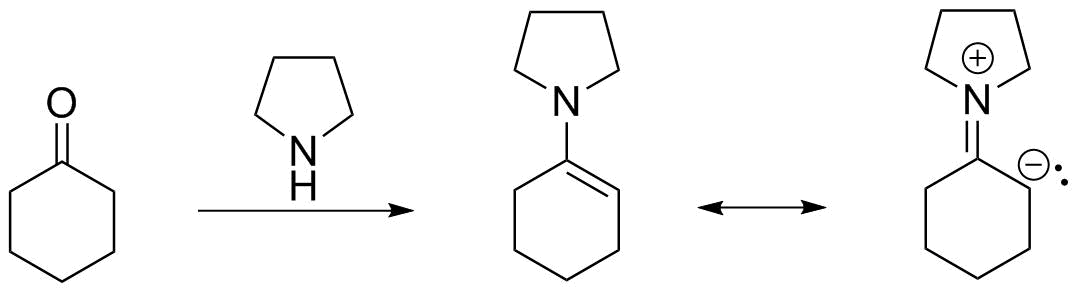
We can see this further by comparing ESP maps of an enolate anion and this enamine:
|
|
|
|
Load the ESP maps Background: White Black Make the surfaces translucent Note the yellow area on the enolate carbon, compared to the orange area on the 6-member rinf of the enamine. This denotes the high electron density and nucleophilic character. |
|
Because the carbon is now nucleophilic, it can be alkylated under milder conditions than required for generating the enolate. Acid hydrolysis restores the aldehyde/ketone functional group.