The Claisen condensation is very similar to the aldol condensation. If you understand why aldehydes and ketones condense, you will easily grasp the principles behind the Claisen condensation.
There are several important features to recognize up from.
- The Claisen condensation requires use of an ester, although the enolate may come from an aldehyde or ketone.
- The selection of a base is critical, because nucleophilic attack on the carbonyl of the ester is a competing reaction. Choosing the base to be the same alkoxide as is present in the ester ensures that the (reversible) nucleophilic attack on the carbonyl results in no net change.
- Crossed Claisen reactions have the same requirements as crossed-aldols: the ester in this case should lack an α-hydrogen.
The mechanism of the Claisen condensation:
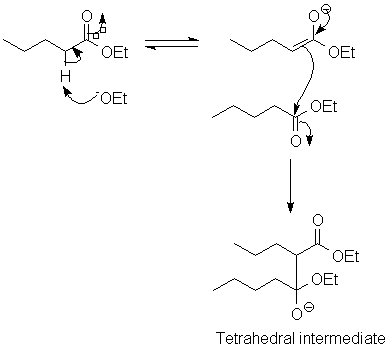
Now, once you arrive at the tetrahedral intermediate, we reach a couple of important differences.
- There is now a good leaving group, so collapse to a carbonyl (not proton transfer) occurs next.
- The product, a β-ketoester (or a β-diketone if the enolate came from a ketone), is unusually acidic, and is deprotonated under the conditions of the reaction. This drives the Claisen condensation to completion.
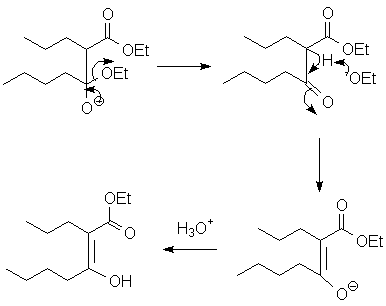
An intramolecular version called the Dieckmann cyclization is an extremely effective way to construct 5- and 6-member rings.
Retrosynthetic analysis
It is a bit more difficult to analyze what the most appropriate reactants would be to form a β-ketoester or (particularly) a β-diketone. This is because two different disconnections are always possible. Consider the following example:
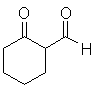
Either carbonyl group could, in principle, come from an ester and thus two different disconnections are possible:
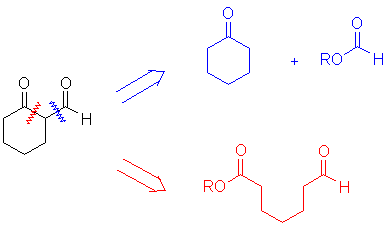
You can anlyze the tradeoffs:
- Does one of the possibilities have an ester that lacks an α-hydrogen? If "yes" then this is a candidate for a cross-Claisen condensation. This example meets this criterion (upper disconnection).
- Are other condensations possible? The lower disconnection above also has an aldol condensation possible, so maybe this is not the best choice.
- Does one disconnection suggest a pair of identical reactants? If the example above had been an ester instead of an aldehyde, the reactant suggested by the lower disconnection would be symmetric and would be an excellent choice for performing a Dieckmann condensation.