The aldol condensation involves three primary steps in the mechanism:
- Formation of an enol or enolate anion.
- Reaction of that enol/enolate with a carbonyl group in another molecule.
- Proton transfer, usually from solvent.
I will use acetone as an example:
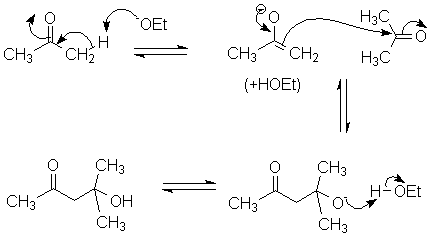
The product has both a carbonyl and an alcohol group; since the chemistry was initially developed with aldehydes the name "Ald-ol" (aldehyde-alcohol) was coined and has been applied to the reaction that forms it.
Several observations are necessary at this point.
- All of the reactions shown above are reversible. You should be able, on your own to predict where each of the equilibria shown above should lie (is K > 1 or is K < 1?)
- You should be able to predict how changes in structure will change the position of the equilibrium. (Examples: more or less bulky R groups in the ketone; ketones vs. aldehydes; electronic effects for electron-donating and electron-withdrawing groups.)
- You should notice that the outcome of a mixture of carbonyl compounds depends on their structure: if there is no α proton, no enolate can form and a cross-aldol condensation is possible. If both can form enolates, and both carbonyl groups are of similar reactivity, mixtures of products will result.
The condensation is usually pushed to completion by dehydration; in some contexts the dehydration may be assumed to be part of the aldol condensation itself.:
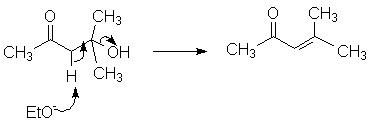
The base-induced elimination is not normally seen with alcohols other than aldols. This reaction probably proceeds via formation of an enolate, followed by loss of hydroxide; formation of the β, β-unsaturated ketone makes up for the poor leaving group ability of hydroxide. (Of course, with acid catalysis the dehydration follows the normal protonation/loss of water/loss of proton mechanism.) Either acid- or base-catalyzed dehydration is helped by increased temperature.
Analysis of products
Trying to figure out how to make a compound requires making mental disconnections. This is much the same as figuring out what reactants to use when making an alkene via Wittig methodology.
Let's take a look at one example:
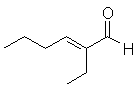
This is an enone, which you can recognize as possibly arising from an Aldol condensation/dehydration sequence. The new C-C bond is the bond that becomes the C=C double bond after dehydration. The carbon next to the remaining C=O was the enolate, and the second C=C carbon must therefore have been the carbonyl of the second molecule.
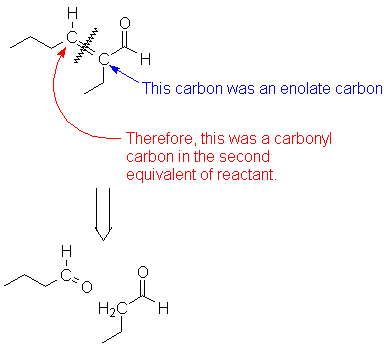
The last thing to do is check to make sure the reactants make sense. In this case, both are identical; good!
If they are not identical you have a problem. Either one of the pieces should lack an α-hydrogen, or you have to find a different way to piece the molecule together.