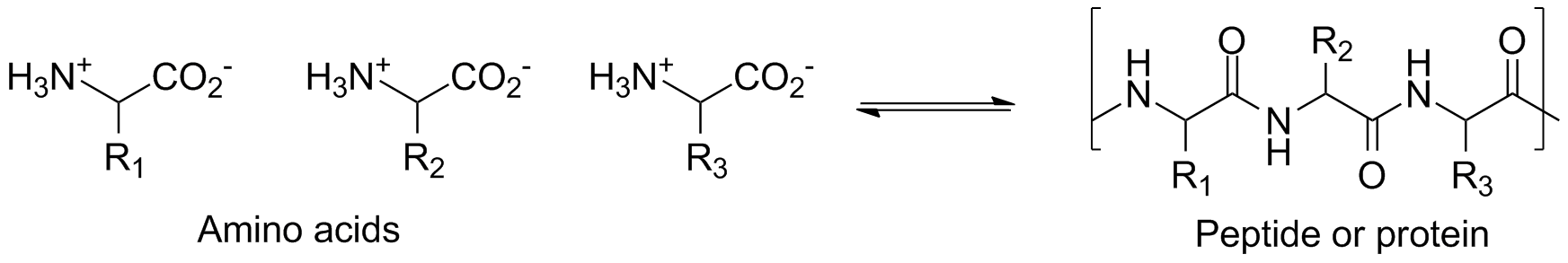
Proteins perform much of the hard work of life processes: they play a major role in structure and physiological function in plants and animals, and they also form enzymes, which are the chemical catalysts that control biological reactions. Conversely, the food we consume contains proteins that must be hydrolyzed to their constituent amino acids before we can efficiently reuse them in our own bodies. Thus, making and breaking amide bonds is a critical piece of life chemistry, and Nature has devised some ingenious methods for applying the chemical principles we know about to allow this reaction to occur in the body at 37ºC and pH = 7.
One important amide-hydrolyzing enzyme is called trypsin. A model of trypsin is shown below.
Click (sequentially) on the following to find the elements of the active site:
Wireframe models for clarity
Highlight Aspartic Acid 102
Highlight Histidine 57
Highlight Serine 195
Zoom into active site
See a substrate in the active site. The serine oxygen is actually bound to a carboxylate as a tetrahedral intermediate in this particular structure.
Ref: Kurinov, I.; Harrison, R. W. Nat. Struct. Biol., 1994, 1, 735-743
The function of these three groups is as follows:
- The aspartate carboxyl group hydrogen bonds to the histidine imidazole.
- This makes this imidazole more electron rich, and a better base.
- The histidine can then remove the OH proton from the serine.
- The serine oxygen becomes more nucleophilic, and can attach the amide CO which is held nearby
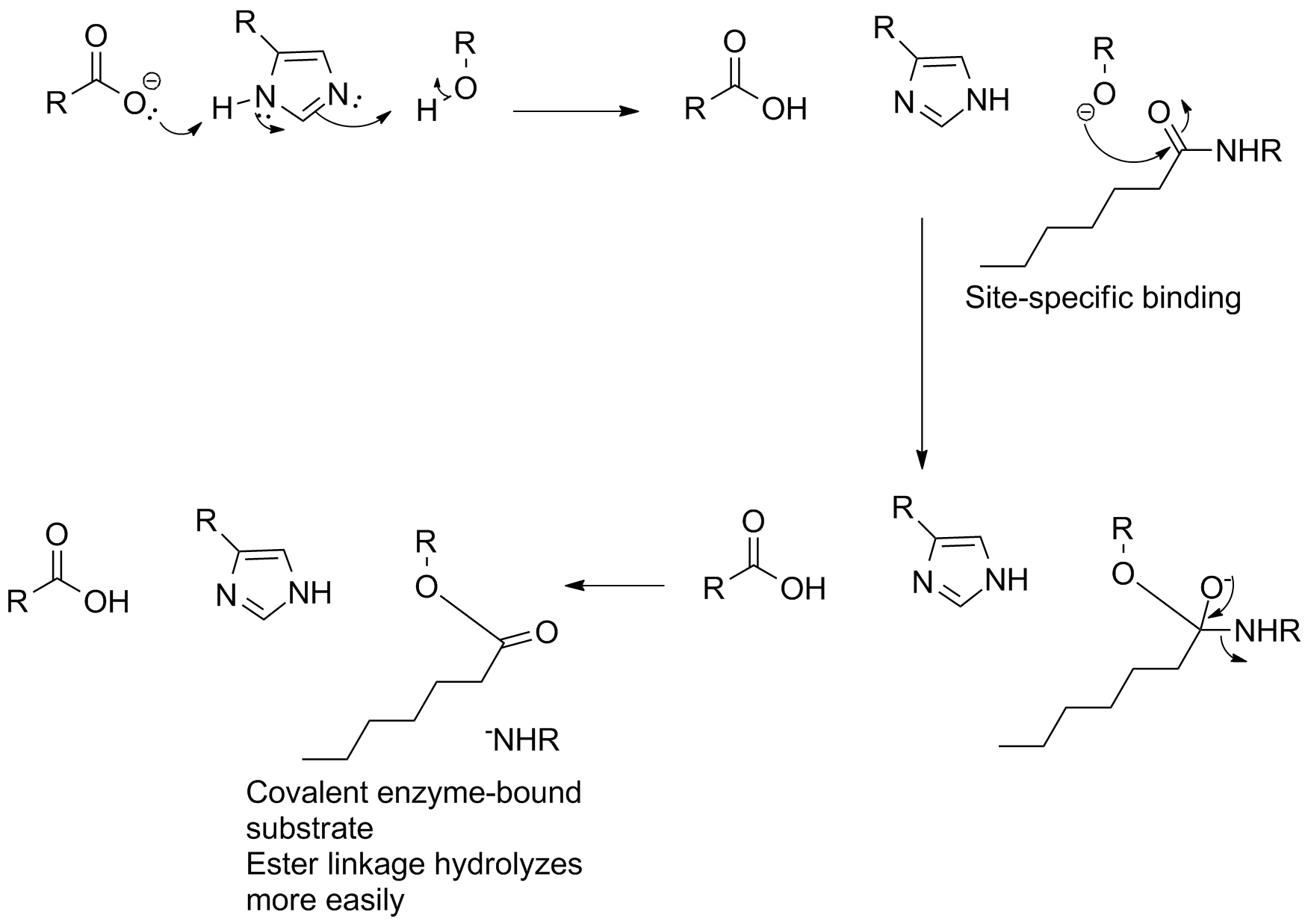
Although the initial nucleophilic attack is slow, the fact that the enzyme holds the substrate in place (and that it controls nucleophilicity via proton transfer) really helps and provides the mechanistic means of overcoming what would normally be a high energy barrier. There is also a "pocket" that stabilizes the negative charge on the tetrahedral intermediate (not shown). The collapse of the tetrahedral intermediate leaves the acyl group on the serine OH oxygen, but now it's an ester that can be rapidly hydrolyzed in a second step.
Essentially, then, this becomes base-catalyzed transacylation from the amide to the serine OH, followed by acid-catalyzed hydrolysis of the ester.