The general mechanism for interconverting carboxylic acids with their derivatives (or converting among derivatives) is:
- Attack by the nucleophile on the acyl carbon to form the tetrahedral intermediate.
- Expulsion of the leaving group accompanied by reformation of the acyl C=O bond.
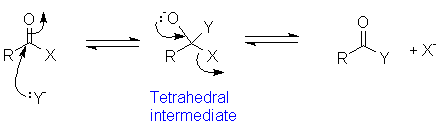
The tetrahedral intermediate is usually unstable and not an isolable compound. Also, the initial nucleophilic attack may be catalyzed by protonation of the acyl oxygen, which makes the acyl carbon more electrophilic. An example is acid-catalyzed formation of an ester:
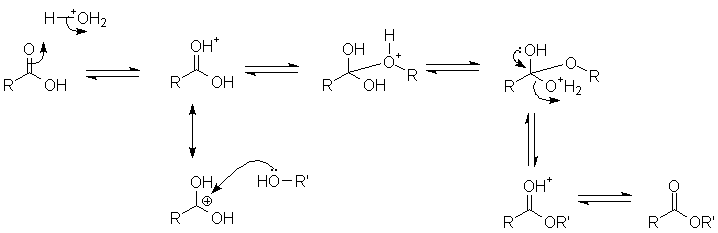
Here, proton transfer also makes a better leaving group.
The general reactivity patterns can be rationalized by which leaving groups are best:
Halide is a better leaving group than a carboxylate; both are better than alkoxide or hydroxide; NR2- is the worst leaving group of all. Changing the leaving groups hydroxide, alkoxide or amide into neutral water, alcohol or ammonia enhances the reactivity. However, the influence of the leaving group on carbonyl electrophilicity is also important. Acid halides react with most nucleophiles spontaneously (activated C=O plus excellent leaving group). Amides require harsh conditions (acid: need protonation to activate the C=O; need to protonate the leaving group; or strong base to generate the best nucleophile possible.)
The electrostatic potential maps of different derivatives can give you a sense for their susceptibility to nucleophilic attack. Remember, blue=more positive, red=more negative. Find and compare the carbonyl oxygens in the following carboxylic acid derivatives.
Backgrounds: Black WhiteAcetyl chloride |
Rate of reaction
|
|
Acetic anhydride |
8.9 x 102 M-1s-1 |
|
Methyl acetate |
8 x 10-2 M-1s-1 |
|
Acetic acid(Note that many nucleophiles--being good Brønsted bases--will deprotonate the acid O-H group. Think about how this will change reactivity.) |
-(Not measurable) |
|
Acetamide |
1.8 x 10-5 M-1s-1 |