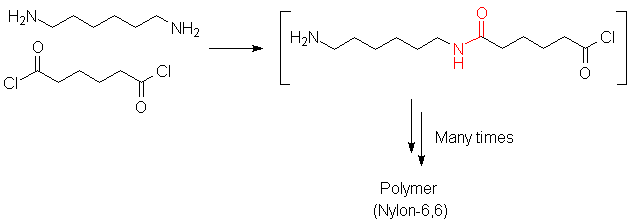
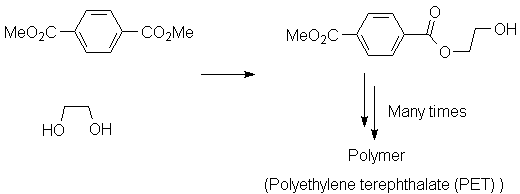
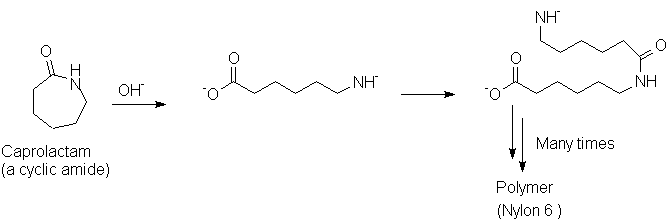
Polyamides and polyesters are two very important classes of synthetic polymers. Production of each type involves chemistry discussed in this chapter.
Backgrounds: Black WhiteEach of these involves the loss of a small molecule (HCl in the case of nylon; MeOH in the case of PET), thus they are called "condensation" polymers. Cyclic molecules may be polymerized in a fashion that does not result in expulsion of a small molecule; an example is Nylon 6, made from caprolactam: