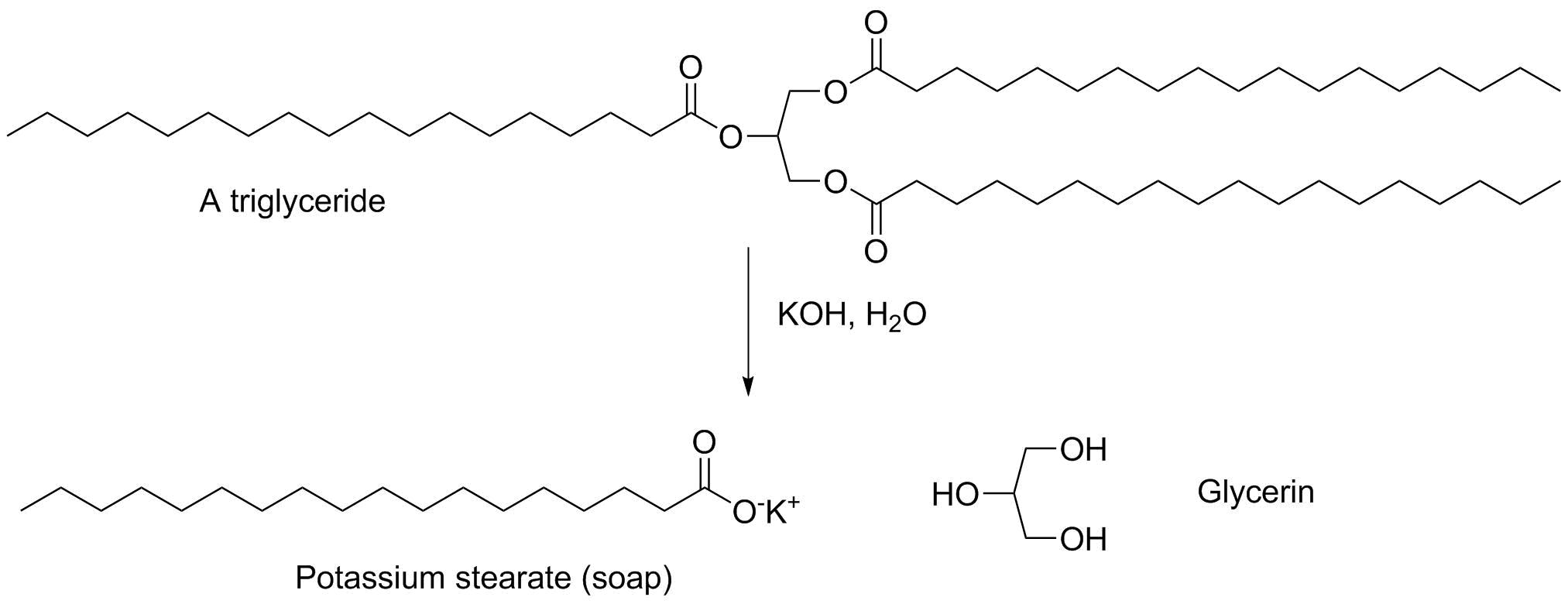
One of the things you know by now is that nonpolar hydrocarbons are immiscible with polar water. One of the earliest technological discoveries was that fats (triesters of the 3-carbon triol glycerin) react with hydroxide (historically, part of the residue of wood ash) to give soap: the carboxylate salt of a long chain carboxylic acid. Soap works by manipulating solvent interactions. The nonpolar ends aggregate in water, placing the polar head groups outward. The structure that forms (a micelle) can dissolve molecules of nonpolar compounds and suspecd them in solution in a polar solvent like water.
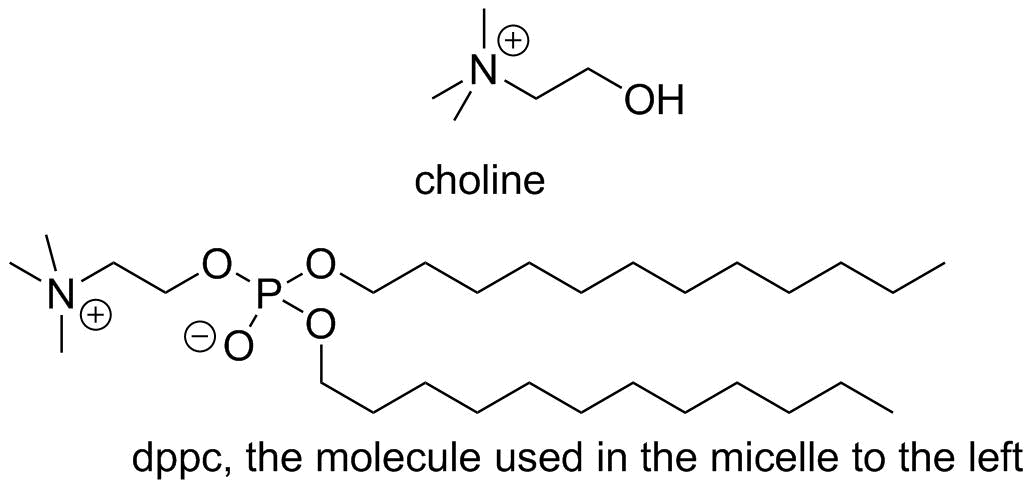
Deemphasize water
Highlight the micelle
Highlight the ammonium nitrogens
Highlight the phosphates
Zoom in on the micelle
If the system has enough of the surfactant, it forms a "lipid bilayer." This is the fundamental chemical structure of cell walls. Cell walls are more complex, though; they contain high proportions of other lipids like cholesterol that help orient the fatty acid chains, and there are embedded proteins (as will as other complex biomolecules) that control membrane transport, sensing and communication.