Naming carboxylic acids is straightforward.
- If the compound (or the primary carbon skeleton) is acyclic, name the alkane for the system that would exist if the carboxylic acid were a CH3 group. Then drop the terminal -e and substitute -oic acid. The carbon in the carboxylic acid group is always carbon 1 (this may make the numbering different from that of the alkane).
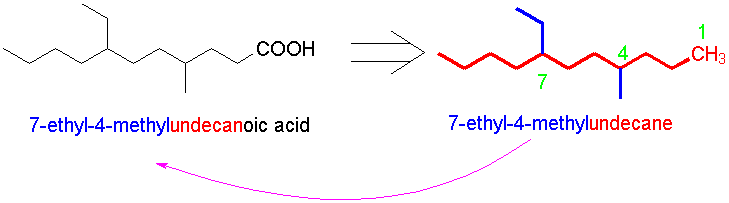
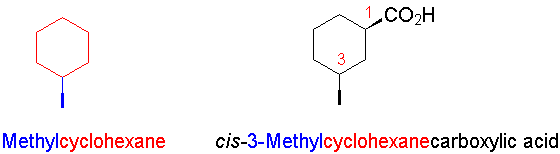
- If another structural feature overrides the carboxylic acid (e.g., a ring), the suffix -carboxylic acid is added to a root name taken by excluding the carboxylic acid carbon. Again, the carboxylic acid defines the numbering, but now C-1 is the carbon bearing the COOH group.
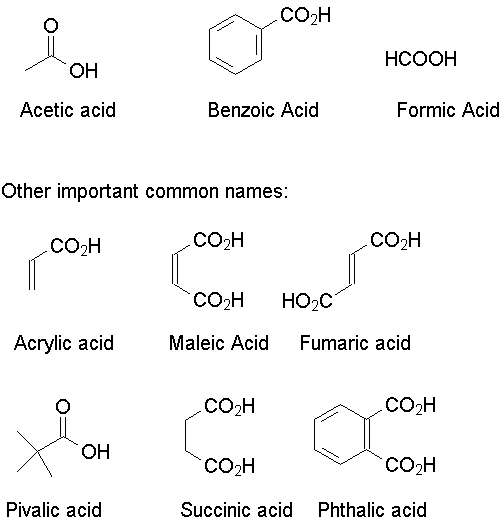
There are a number of carboxylic acids for which the common name has been accepted by IUPAC:
Salts of carboxylic acids are named using the positive ion, then the name of the acid modified by dropping -oic and adding -ate.
Thus, acetic acid forms a salt sodium acetate, Na+CH3CO2-.