The nature of the R group also plays an important role. There is no direct resonance interaction, but the inductive effect of an electron poor or electron rich R group significantly changes the pKa of the acid. Compare the following structures and pKa values. Look at the electrostatic potential maps to see another measure of this trend. Find the carbonyl oxygen (red) and the acidic proton (blue) and see how the intensity of the color varies with pKa.
Acid,Structure, pKa |
Structure, ESP MapLoad ESP mapsBackground: Black White |
Trifluoroacetic acid, F3CCO2H0.23 |
|
Chloroacetic acid, ClCH2CO2H2.85 |
|
Formic acid, HCO2H3.75 |
|
Benzoic acid, PhCO2H4.19 |
|
Acetic acid, CH3CO2H4.75 |
|
Pivalic acid, (CH3)3CO2H5.03 |
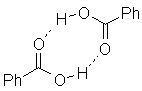
A final effect of the weak acidity is that carboxylic acids are excellent H-bond donors. They actually have a pronounced tendency to exist as H-bonded dimers. This is reflected in their spectroscopic behavior, particularly in the infrared spectrum.
An example is the H-bonded dimer of benzoic acid:
 |