Carboxylic acids are, by definition, Brønsted acids that can donate a proton to ther
species with lone pairs.
![]()
They are generally weak acids (vs. the hydronium ion), so the equilibrium above lies far to the left. For something like acetic acid, the pKa is 4.75, so K for the above expression is 10-4.75, which is about 0.00001. (You have probably considered in General Chemistry the problem of vinegar--a 5% to 8% by weight solution of acetic acid in water. This works out to be approximately 1 M; the expected, and observed, pH is 2.4.)
However, carboxylic acids are much more acidic than alcohols (pKa =
16-18). One explanation for this is the resonance delocalization afforded by the
carbonyl group:
Top: R=tert-Bu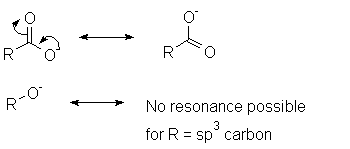 Bottom: R=tert-Bu Turn on ESP maps |
|
Use the structures below to examine the impact of structure on C-O and C-C bond lengths.
| Turn on the distance monitor with the button below, then select a set of
bonds. Then click on any two atoms; the distance will be displayed on the status bar of
your browser.
Turn on
distance monitor |
(This is not the entire story; the electron withdrawing nature of the CO group
helps, compared to the electron donating nature of the R group. A hair-splitting
question is whether the resonance in the carboxylic acid is as important as the resonance
in the carboxylate.)
Go on to Acidity Page 2.