How do we figure out how to propose a synthesis, when processes like the Claisen condensation, the acetoacetic acid synthesis and malonic acid synthesis have many different reactions in sequence? The most important skill is to recognize the general structural features of the product, then to "disconnect the pieces" and propose good starting materials.
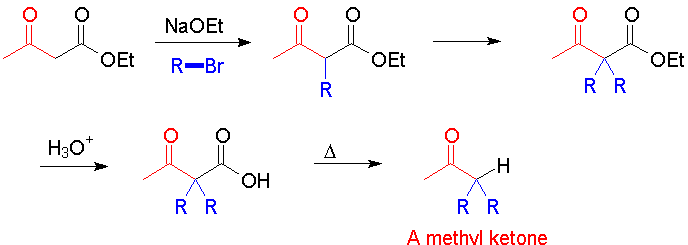
Note that the acetoacetic acid synthesis makes a methyl ketone. You may have one or two alkyl groups, and they need not be identical.
So how would we use this to make cycloheptyl methyl ketone? First recognize the methyl ketone and the 3-carbon unit that would come from an acetoacetic ester (in red). Then, look at the pieces that are connected to this fragment: the C-R bonds (above and below, blue) can then be found in the product. These are formed from an alkyl halide, so it's easy to see that the (single) other reactant in this case is 1,5-dibromopentane.
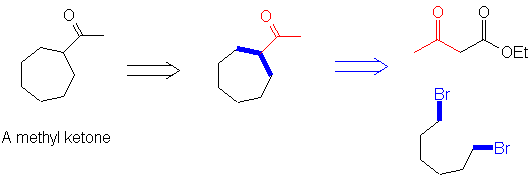
So the keys to constructing a synthesis are:
- Know the general features of the reaction chemistry. This is one area where memorization is necessary.
- Identify the key structural feature for a reaction you know in the desired product.
- Mentally disconnect the bonds that would be formed by the reaction you choose.
- Draw appropriate reactants.
- Make sure the specific compounds you chose have no incompatibility with your reaction conditions (e.g., the presence of an OH group if you need to add a Grignard; or use of a 3° alkyl halide to perform an SN2 substitution).