acetylcoa.mol |
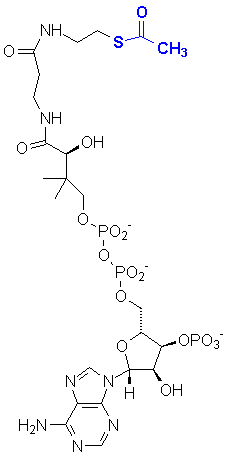 |
Coenzyme A is an important template molecule for biological chemistry based on aldol condensations. For example, the acetylated version, acetyl-coenzyme A (AcCoA) is important in the citric acid cycle.
The S-acetyl group can be enzymatically deprotonated and will participate in reactions resembling aldol and Claisen condensations. For example, two molecules can react to form acetoacetyl-CoA:
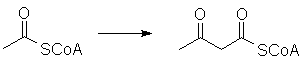
In fatty acid synthesis, the keto carbonyl is reduced, dehydrated and reduced again to form a butanoyl group. Additional Claisen-type reactions at the acyl group extend the chain by 2 carbons at a time.
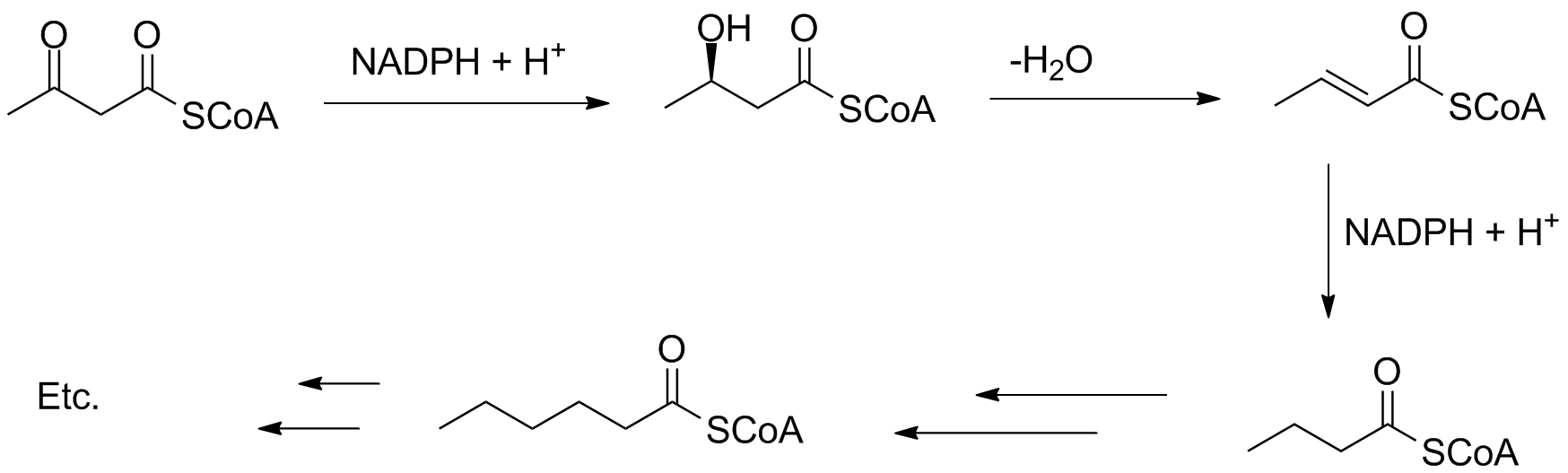
The chain growth can be coded to stop at a certain length, and the reduction steps can be skipped; this often happens in the biosynthesis of mono- and polyunsaturated fatty acids.
However, the chain can also be extended through multiple Claisen-type reactions:
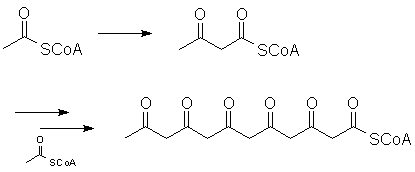
Look at what can happen when this "polyketone" curls around on itself:
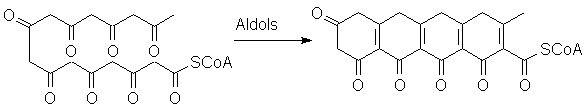
You should recognize the possibility of proton transfers, leading to aromatization of several rings:
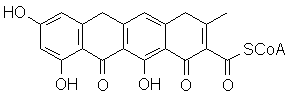
In actuality, there are minute differences in the order of steps, and other reactions are performed to add or cleave other alkyl groups and change oxidation state at specific positions. However, you should be able to recognize the "blueprint" of the structure above in the structure of the antibiotic tetracycline, itself a metabolite made by the one-celled organism Streptomyces viridifaciens:
Many natural products contain this motif. For another example, look at erythromycin, produced by a different strain of Sterptomyces (S. erythrens):