Aldol Condensation Chemistry in Action
The Robinson annulation is two enolate condensations in tandem. The first is a conjugate addition of one enolate to an a,b-unsaturated ketone:
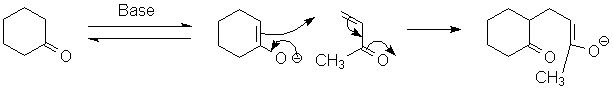
This forms a new enolate. Normally this is not reactive (it would lead to an unfavored 4-member ring):
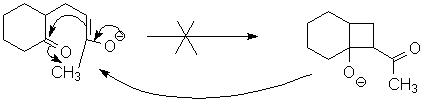
However, proton transfer can occur to make the carbon on the opposite side of the carbonyl nucleophilic. (Work this problem: how many different isomeric enolates are possible at this stage?)
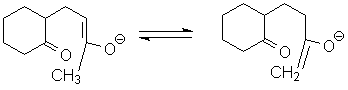
This new enolate will now close to form a 6-member ring, which is quite favorable:
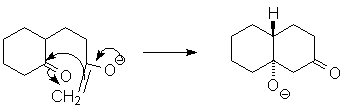 Background: Black White |
Push
the button here to see the parts of the aldol condensation. The blue is the enolate,
and the red is the carbonyl. Push the second button to illustrate the bond formation process. Reload the molecule. |
Finally, to push the equilibrium to the product, dehydration of the aldol adduct occurs, making a (conjugated) double bond.
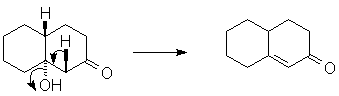 In this case, assisted by base (hydroxide); essentially a retro-Michael reaction. Under acid-catalyzed conditions, a similar E1-like dehydration would occur, driven by the stability of the α, β-unsaturated carbonyl. You should be able to write both base- and acid-catalyzed mechanisms for the dehydration. |