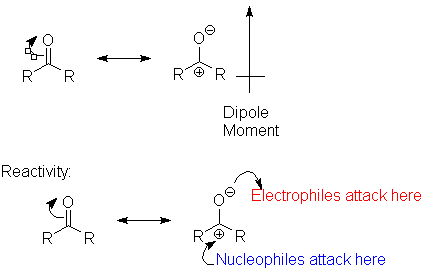
The C=O bond is highly polarized toward the oxygen. This is primarily an effect of the oxygen atom's electronegativity; however, resonance effects accentuate this over what is seen in an ether:
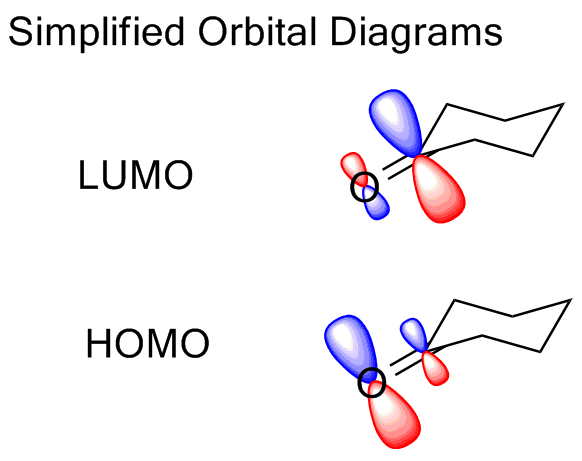
One way we can further understand the reactivity uses the results of molecular orbital calculations. The highest occupied molecular orbital (HOMO) shows where electrophiles will attack; the lowest unoccupied MO will show where nucleophiles will attack. Alternatively, the relative charge distribution diplayed in an electrostatic potential map can tell us similar things.
Load LUMO
Load HOMO
White backgrounds
Black backgrounds
- The composition of the pi bond, arising from interaction of p orbitals on the sp2-hybridized carbon and oxygen atoms.
- In the HOMO, the density is greatest on oxygen, reflecting the polarization of the bond. Most of the electron density in the pi bond will reside on oxygen, rather than carbon. (Compare this to the equal distribution in a C=C pi bond.)
- In the LUMO, the biggest part of the MO is on carbon. If we interact with a Lewis base, the carbon atom will be the primary point of interaction because of this, and the orientation of this orbital governs the trajectory of attack of the nucleophile.
Load ESP surface
Now load the electrostatic potential map for cyclohexanone (click link to load).
Blue = positive potential;
Red = negative potential.
The most important reactivity feature of the carbonyl group is that (electon-rich) nucleophiles attack at the carbonyl carbon to form new bonds.
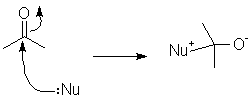
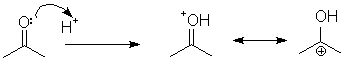
Note when you load the ESP map, that the molecule as a whole is more blue (positive), centered on the added proton and the carbonyl carbon. Any Lewis acid has this effect of binding to oxygen, and activating the carbonyl carbon for nucleophilic attack.
Load ESP surface
(Why, do you think?)
Also, the steric congestion is far less at an aldehyde than a ketone.
Load ESP surface