We can use the resonance description of the C=O bond to treat nucleophilic attack as a primarily electrostatic phenomenon: attraction of the (negative) electrons on the nucleophile to the positive end of the polarized C=O bond:
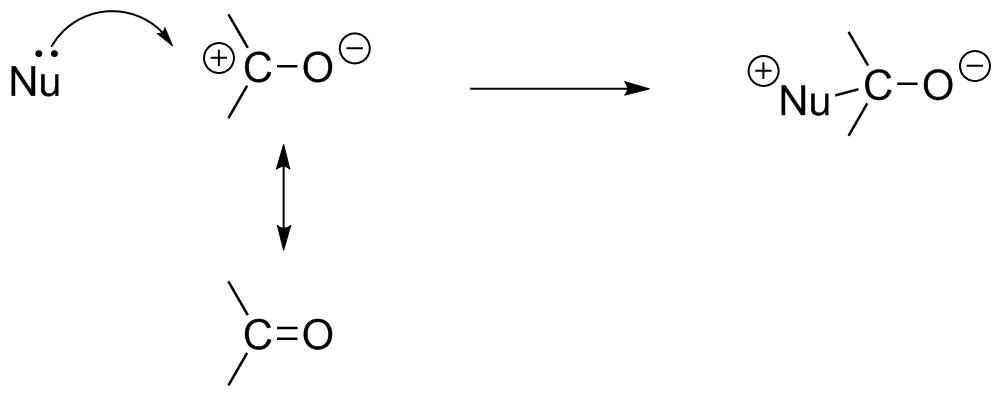
Recognize that this is a simplistic model. A more sophisticated approach is to recognize that this will be driven by a molecular orbital process that involves making a new bonding MO (as well as a new antibonding MO) from two MOs of the reactant: the HOMO of the nucleophile (describing the electrons used to make the bond), and the LUMO of the carbonyl, which describes where and how elections can interact.
A seminal structural study identified the structure of the natural product protopine (right) as a model for the process. The conformation of the large ring holds the nitrogen in close proximity to the carbonyl. We can see the interaction between the nitrogen lone pair and the carbonyl LUMO:
Show the Lone Pair on Nitrogen
Show the LUMO
Background: Black White
Model type: Wireframe Ball-and-stick