An early observation in substitution chemistry led to a mechanistic conundrum.
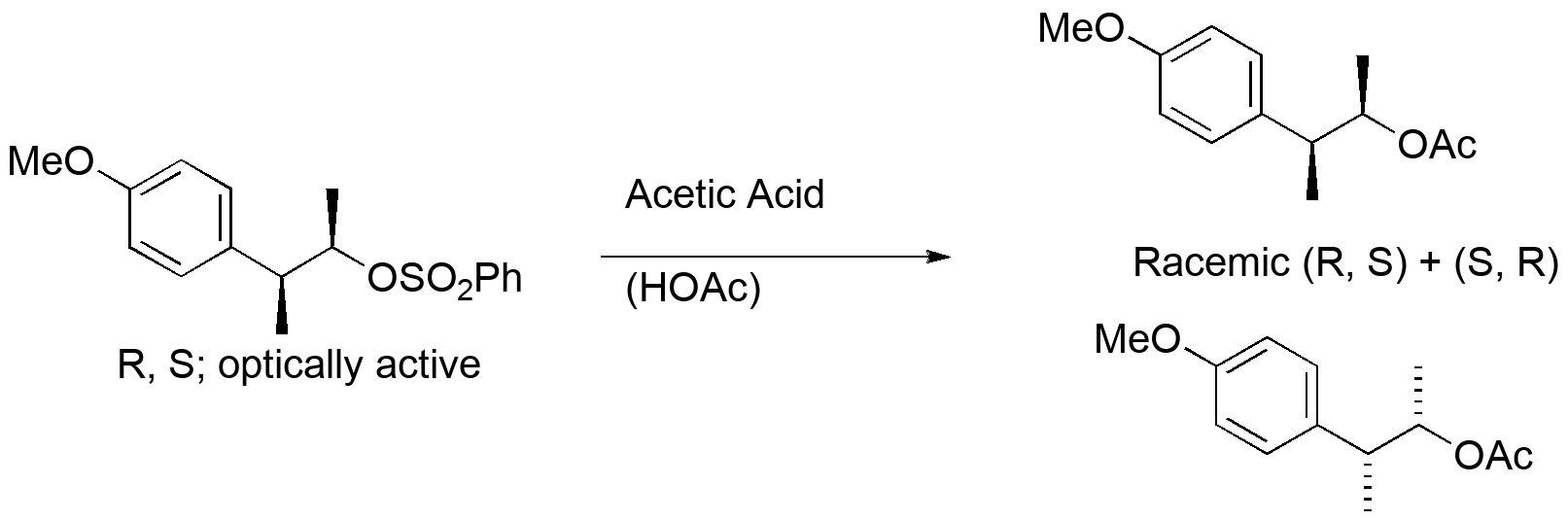
Two things seem wrong given what we know about nucleophilic substitution. The methyl group at C2 should not change configuration at all, and the change at C1 should either be inversion by SN2 to give (S,S), or we have an SN1 process that should give a mixture of (R,S) and (S,S).
The solution is to consider that the aromatic π system can be an internal nucleophile. Particularly a ring with an electron donor will undergo the equivalent of a Friedel-Crafts alkylation; the donor will direct para to itself (there isn't enough chain length to reach the ortho position). Look at 3-D models (and build your own!) of the reactant and the intermediate "phenonium" ion:
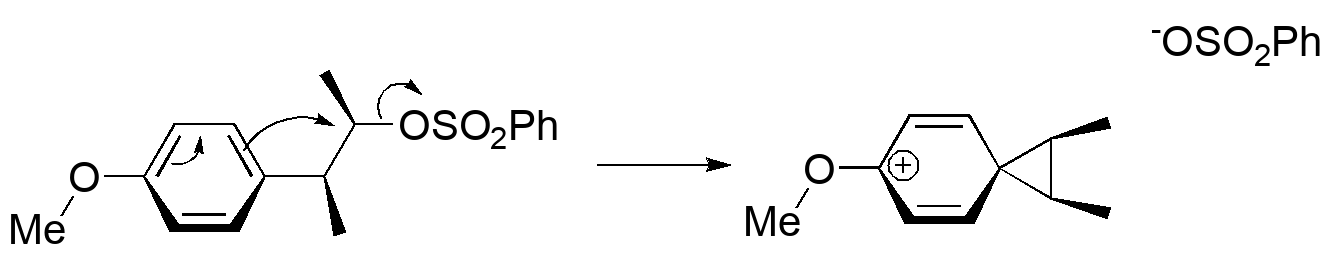 We can observe that this even happens in the parent (nonmethylated) 2-phenethylsulfonates using 13C labeling experiments. The amount of rearrangement depends on the donor/acceptor properties of the benzene ring. This observation fits with a series of rearrangements of alkyl-substituted benzenes under conditions where an electrophile is generated in the molecule.
We can observe that this even happens in the parent (nonmethylated) 2-phenethylsulfonates using 13C labeling experiments. The amount of rearrangement depends on the donor/acceptor properties of the benzene ring. This observation fits with a series of rearrangements of alkyl-substituted benzenes under conditions where an electrophile is generated in the molecule.