Show pz orbitals or Orbitals off Show the highest energy MO, E=-0.246 eV Show the second highest-energy MO, E=-0.246 eV Show the lower energy MO, =-.359eV Black background White background Spacefilling model Wireframe Ball & Stick |
The
buttons are listed in
descending order of energy. These represent different ways the p atomic orbitals mix to form MOs. Another representation is below: 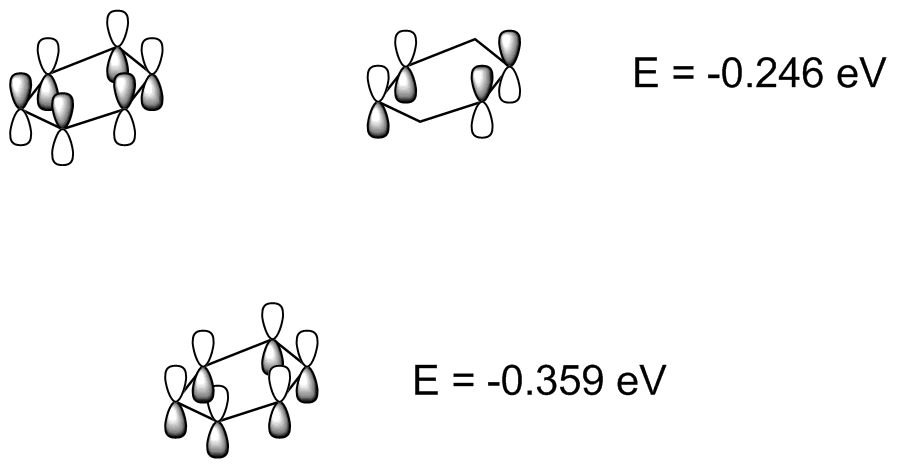 Compare this to the resonance picture, which correctly describes all carbons as being identical,and makes all the C-C bonds identical, but misses that there are two HOMOs of identical energy but one more stable filled MO to account for the six electrons. 
(I have not shown any of the empty, antibonding MOs. See Fig. 15-4 in Vollhardt & Schore.) |
|
While we are not covering the underlying principles of how we arrive at the molecular orbitals, you can get more background at these links: The Wikipedia page on a simplified method for calculating π MOs A Simple Huckel MO calculator (requires an active Java plugin) A Javascript-based SHMO calculator that requires an understanding of the mathematecal approach outlineed in the Wikipedia page | |