The molecular orbital description of bonding in dienes is an extension of the jump from alkene to allyl--one more sp2 center with one more p orbital is involved. There is an increase in the net stability of the system versus isolated double bonds, though the HOMO is now higher in energy than the alkene HOMO.
Show pz orbitals or Orbitals off Show the highest energy MO 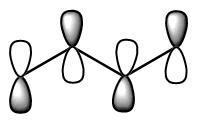 Show the LUMO 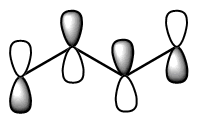 Show the HOMO 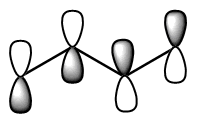 Show the fully bonding pi MO 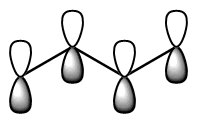 |
The
links are listed in
descending order of energy; the lowest is the most stable. The bottom 2 are occupied (4 π electrons total). We can draw a number of resonance forms but we start to get to a point where resonance does not really help us any more. There are a couple observations that come from the MO analysis:
Black background White background Spacefilling model Wireframe Ball & Stick |