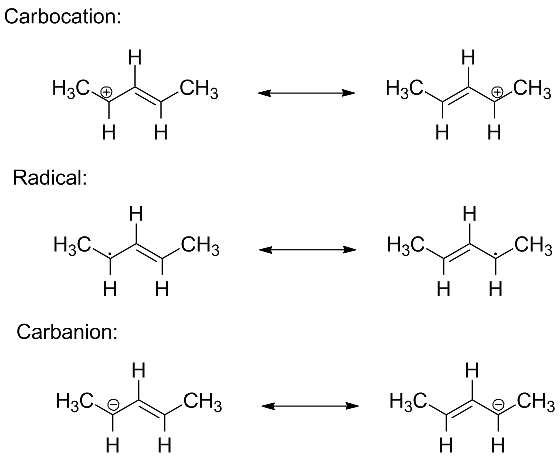
The molecular orbital description of bonding in allyl systems is almost identical regardless of whether we have a radical, a carbocation or a carbanion.
In all cases, the three unhybridized p atomic orbitals combine to make a fully bonding MO, a non-bonding MO (with a node on the central carbon), and an antibonding (fully out of phase) MO. We will populate these three MOs with either 2 electrons (in the allylic carbocation), 3 electrons (in the radical) or 4 electrons (in the carbanion).
The three MOs for the dimethylallyl system are shown below. Click on the radio buttons to visualize each molecular orbital.
|
Show pz orbitals or Orbitals off Show the highest energy MO Show the nonbonding MO Show the fully bonding MO |
The
buttons are listed in
descending order of energy. For the carbocation, the energies in eV are: -4.33(empty) -8.70 (empty) -13.6 (full-2 electrons) For the radical, they are: 1.68 (empty) -4.50 (singly-occupied) -7,85 (full-2 electrons) (the extra electron destabilizes every energy level a little bit) For the anion, they are: 7.66 (empty) 2.54 (full-2 electrons) -1.32 (full-2 electrons) |
Compare the MO picture with the resonance description below and identify the parallels: