Living on earth presents a significant chemical challenge. The molecule we rely on to drive many of our biochemical processes &emdash; molecular oxygen — is itself so reactive that it can attack our cellular structures. This derives from the fact that the O2 is a ground state triplet, meaning that it has two unpaired electrons. (See the Wikipedia page on O2 for an explanation.) It behaves as a radical and is a quite efficient initiator of a range of radical reactions, both chain reactions and otherwise.
As you may be aware, fats and fatty acids are significant components of biological systems. They are critical aspects of structure in the cell membrane, and serve a number of chemical functions within the cell including energy storage. Many of the long chain fatty acids have one or more double bonds. While many alkenes are prone to O2-initiated polymerization (the word "olefin" comes from the observation that these compounds were "oil-makers") you are also aware that they have a reactive allylic C-H bond. This bond is weakened precisely because of the allylic resonance of the radical formed by C-H abstraction.
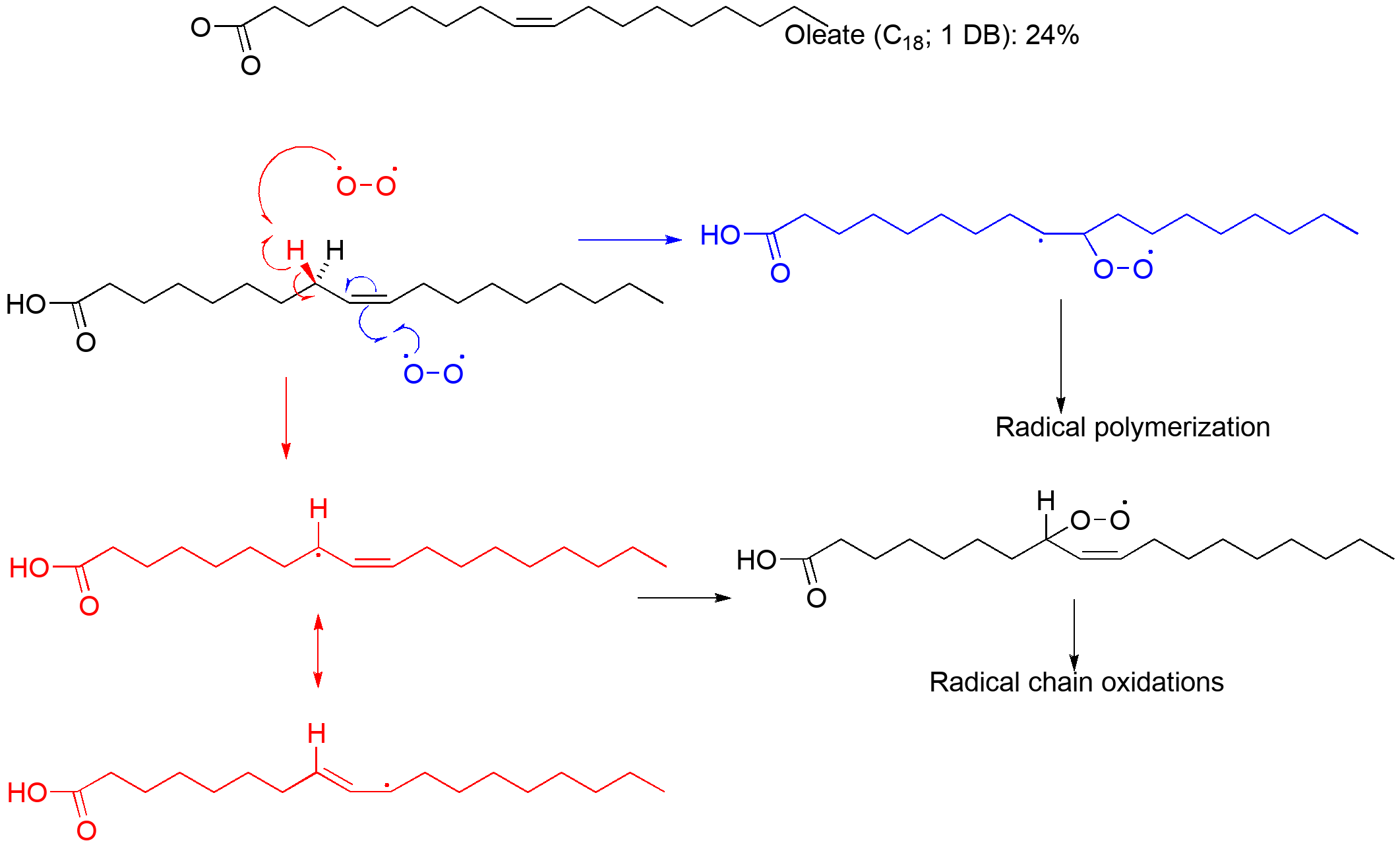
The C-H bond in these Z alkenes is only worth 82-85 kcal/mol; one between two double bonds is only 76 kcal/mol!
Our biology actually takes advantage of this reactivity in generating compounds that respond to this oxidative damage and mediate an inflammatory repair response. Both leukotrienes and prostaglandins are chemical messengers that trigger pieces of the immune response to damage.
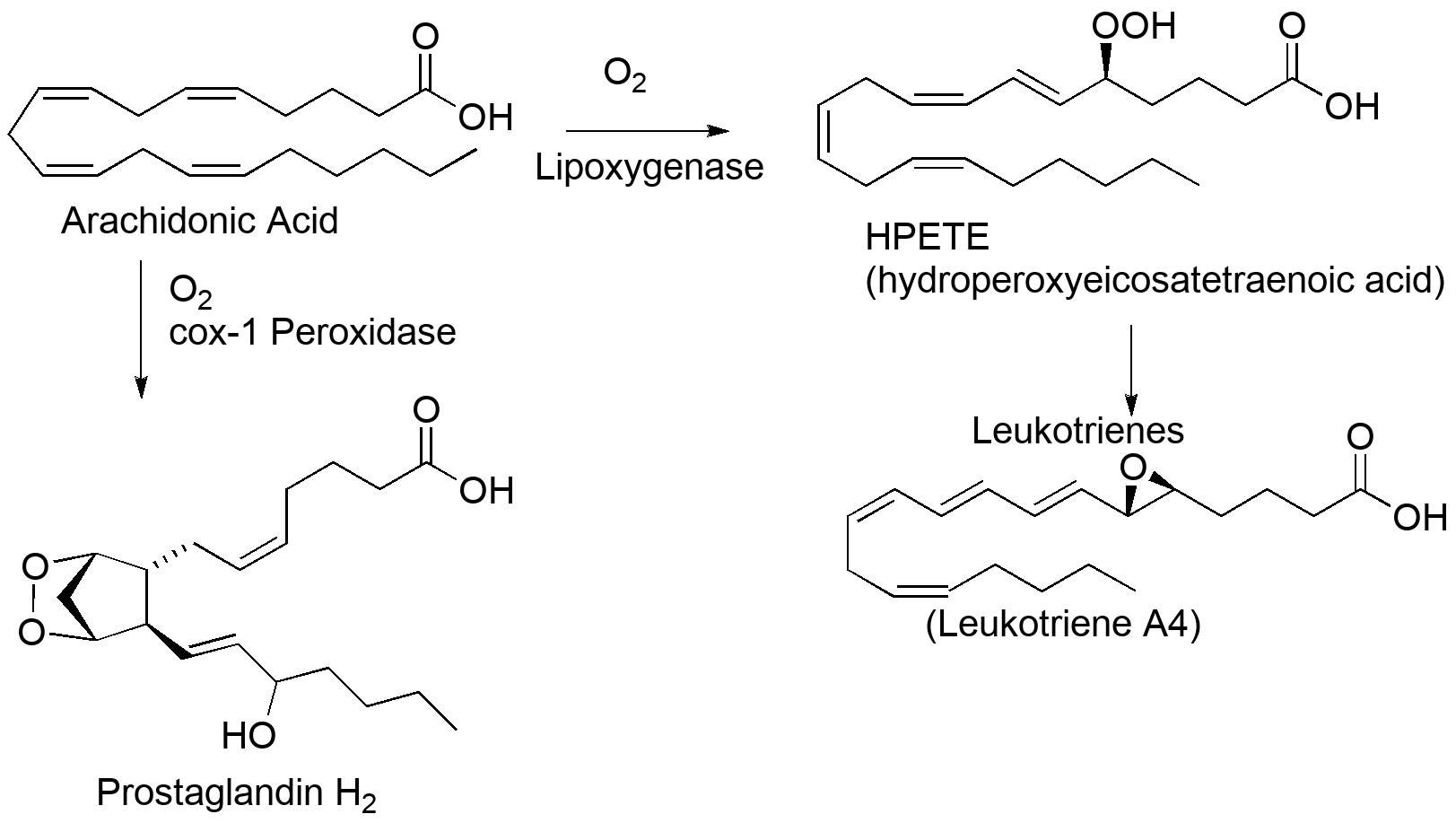
Aspirin and other NSAIDs work by inhibiting the cyclooxygenases and shutting down the cascade of prostaglandin-making reactions. The damage is still there, but the drugs moderate the inflammation that is often the source of pain. It's worth noting that both prostaglandins and leukotrienes are involved in a range of biochemical signaling (including in the reproductive system), and even in the case of pain and inflammation, the symptoms are related to the necessary repair functions we depend on.