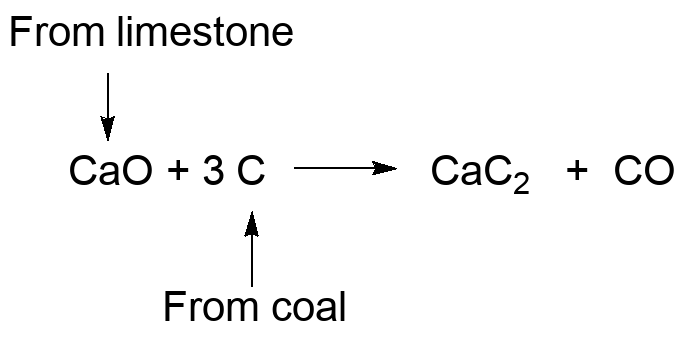
Calcium carbide is a diacetylide, and a ready source of the acetylide anion synthon. We've seen it used to make long chain alkynes, and reaction with water forms acetylene and Ca(OH)2.
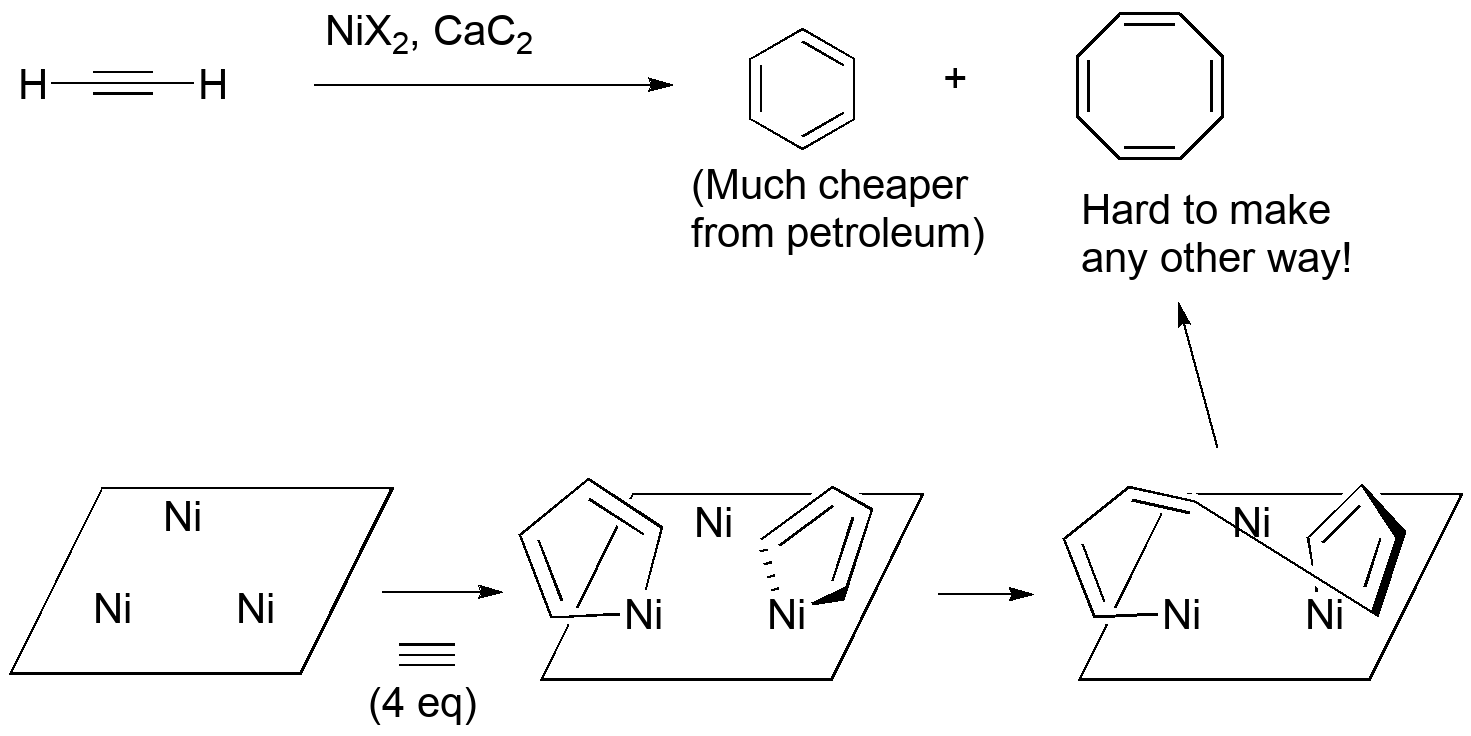
(Fun fact: Dr. Gable did his Ph. D. thesis studying the catalyst and found evidence that it's a complex organic framework assembled from the acetylides that supports a number of reduced Ni atoms. See paper in Organometallics.
One application was discovered in the 1990s. Another metal-catalyzed process called "olefin metathesis" essentially switches ends of two molecules with double bonds. When this is done with cyclooctatetraene, the rings open up and link together, resulting eventually in a high polymer. This "Ring-Opening Metathesis Polymerization" or "ROMP" results in a long chain of conjugated double bonds. This is conductive, and its discovery was awarded the Nobel Prize in 2000.
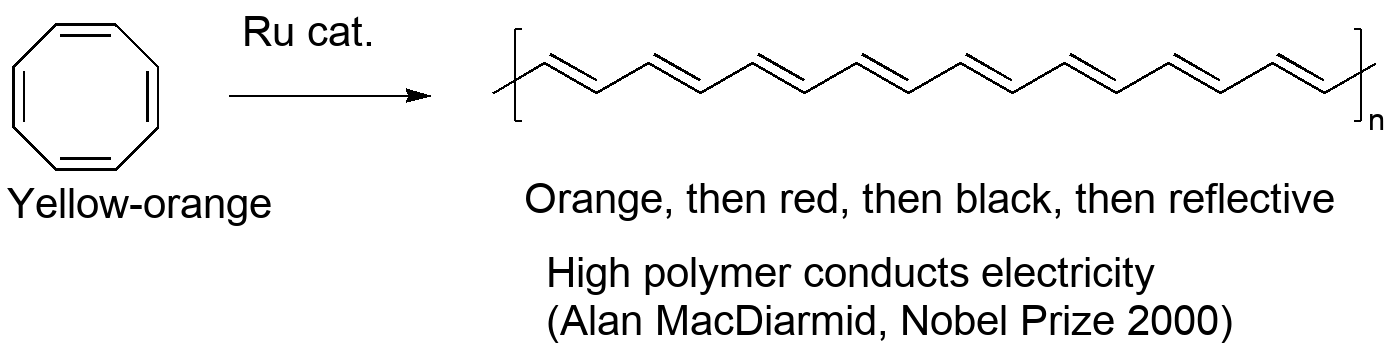
A paper with a picture of a sample of conductive polyacetylene: https://iopscience.iop.org/article/10.1088/1757-899X/54/1/012019/pdf