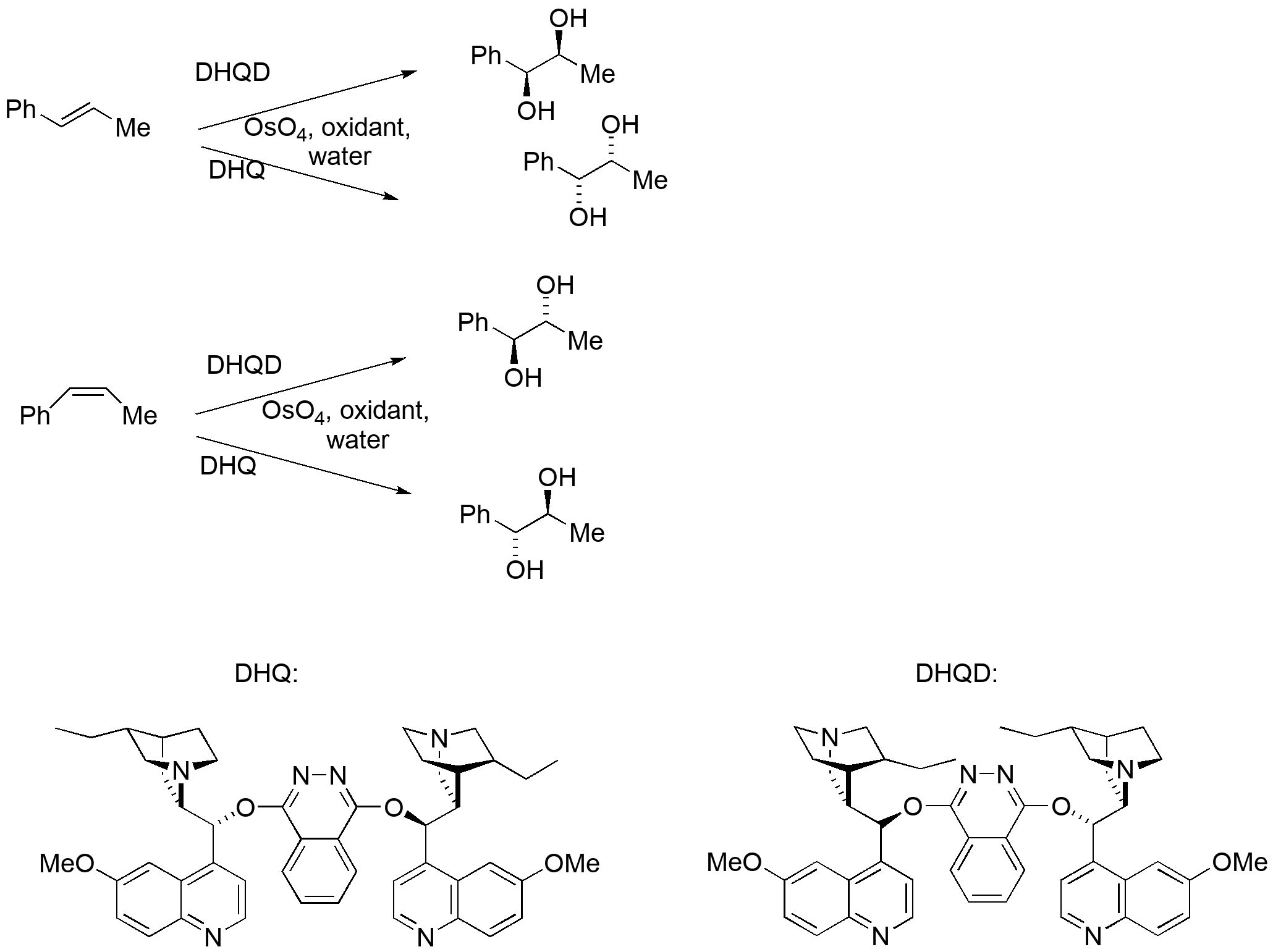
Osmylation of alkenes has become a critically important method of functionalizing an alkene due to two discoveries: 1) the reaction rate is accelerated by addition of amines that bind to Os; 2) use of chiral amines (particularly those derived from cinchona alkaloids such as quinine and quinolidine) allow not just high stereocontrol (syn addition), but also complete enantiocontrol — any absolute stereochemistry at the diol can be prepared based on the configuration of the double bond and the alkaloid ligand chosen.
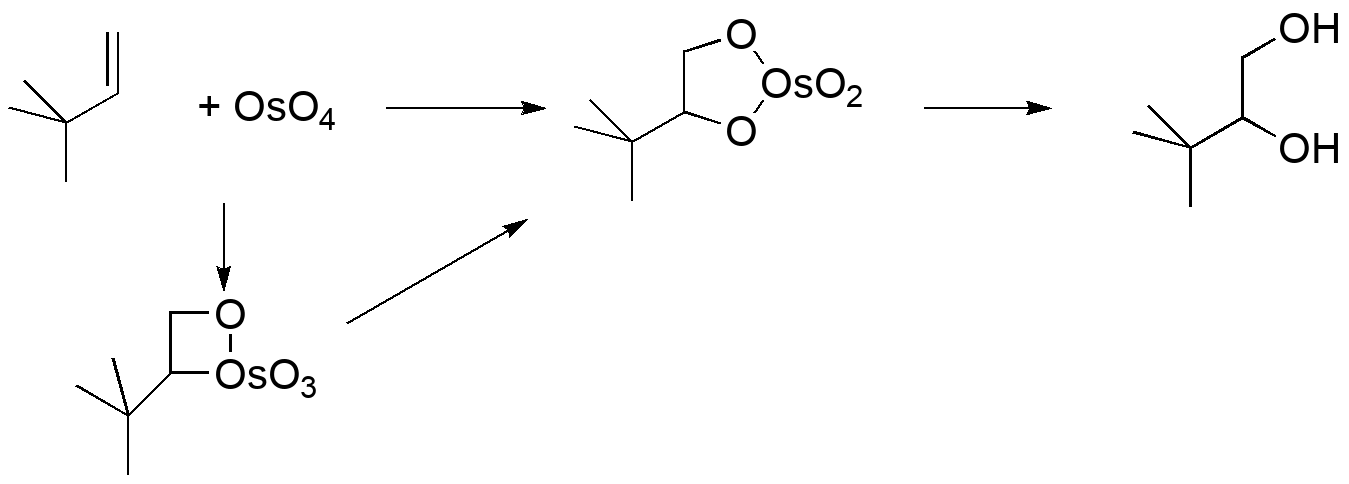
Computational modeling can identify transition states for each step in both mechanisms: